- E-mail:BD@ebraincase.com
- Tel:+8618971215294
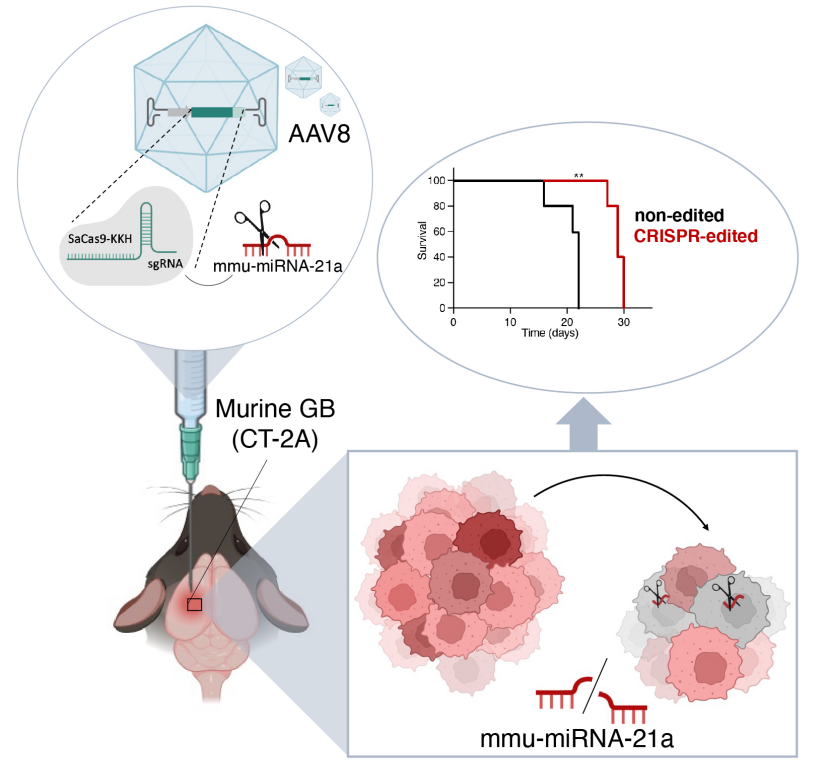
On November 19, 2024, the team led by Erik R. Abels from Leiden University Medical Center in the Netherlands published a research paper titled “CRISPR targeting of mmu-miR-21a through a single adeno-associated virus vector prolongs survival of glioblastoma-bearing mice” in Molecular Therapy. In this study, the researchers used a single adeno-associated virus vector (AAV8) carrying a variant of SaCas9 (a staphylococcal Cas9 homolog) called SaCas9-KKH, along with guide RNA (sgRNA), to edit the miR-21a gene in tumor cells of a glioblastoma (GB) mouse model. This approach led to a reduction in mmu-miR-21a levels in the brain, which suppressed tumor growth and improved overall survival, resulting in beneficial anti-tumor effects in glioma-bearing mice.
Efficient In Vitro Editing of mmu-miR-21a in Mouse Glioblastoma (GB)
The researchers utilized CRISPR-Cas9 gene editing technology, a revolutionary genetic engineering tool capable of precisely modifying the genome. To target mouse miR-21a, the team selected Staphylococcus aureus Cas9 homolog (SaCas9-KKH) and specific guide RNA (sgRNA). Eleven different sgRNAs were tested to identify the most effective one for reducing miR-21a expression. Experiments were conducted in mouse glioblastoma cell lines (such as GL261 and CT-2A) by co-transfecting the SaCas9-KKH expression plasmid and sgRNA expression plasmid, or using a single plasmid that co-expressed both SaCas9-KKH and sgRNA (referred to as an “all-in-one” plasmid). qRT-PCR measurements showed that after co-transfection of specific sgRNAs (such as sgRNA3) with SaCas9-KKH, the expression level of miR-21a-5p was significantly reduced. Sanger sequencing and next-generation sequencing (NGS) analysis revealed insertions or deletions (indels) at the miR-21a gene locus in the CRISPR-edited cells, indicating successful genomic editing. The CRISPR-edited cells showed reduced proliferation rates, which may be related to the decrease in miR-21a levels.
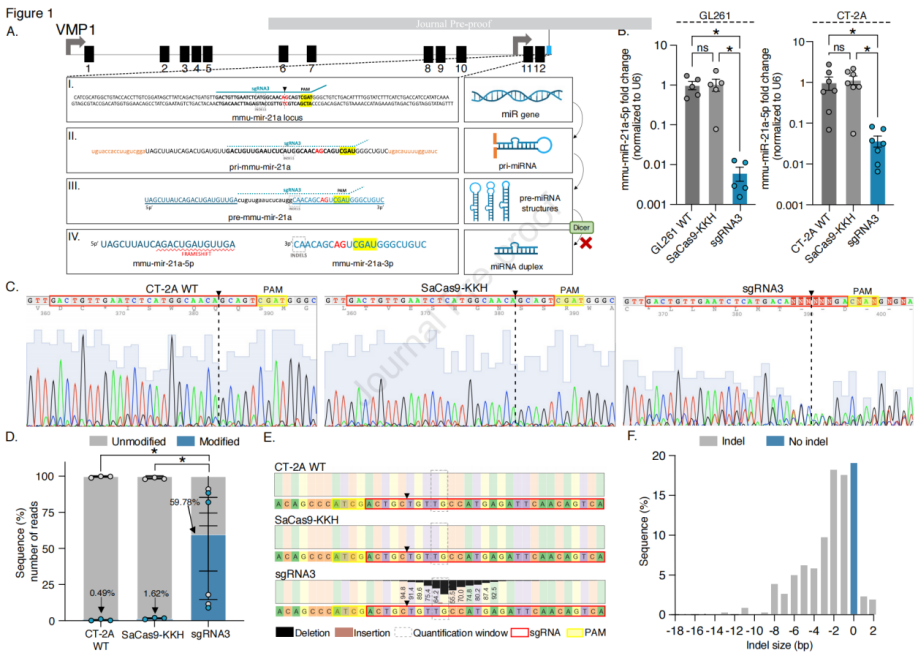
Figure 1: Efficient In Vitro Editing of mmu-miR-21a in Mouse Glioblastoma (GB)
In the experiment, a plasmid was constructed to simultaneously express SaCas9-KKH and sgRNA, with the CMV enhancer and CAG promoter fused to express SaCas9-KKH. The plasmid also contained a 3x FLAG tag, a 2A peptide (p2A) sequence, and green fluorescent protein (GFP), along with sgRNA3 driven by the U6 promoter. In vitro transfection was performed to evaluate the editing effect of the CRISPR-Cas9 system on mmu-miR-21a, including changes in mRNA levels and effects on cell proliferation. After transfection, cells were sorted into GFP-positive and negative populations, and the expression of the 3xFLAG tag and mmu-miR-21a-5p levels were further analyzed. The results showed that compared to the control group, mmu-miR-21a levels were significantly reduced in the CT-2A cells transfected with the single plasmid, and cell proliferation rates were also lower. As plasmid dosage increased, transfection efficiency improved, and the expression level of mmu-miR-21a-5p decreased. However, the highest dose (1600 ng) did not show stronger inhibition of mmu-miR-21a-5p expression due to increased cell death. In GFP-positive sorted cells, mmu-miR-21a-5p expression was significantly reduced, and disruptions were observed in the genomic sequences of these cells. Sanger sequencing and next-generation sequencing (NGS) analyses confirmed the genomic editing effects, including the generation of indels and assessment of off-target effects. The results showed that SaCas9-KKH editing in the mouse genome had minimal off-target effects, with very few modified reads detected at the top five predicted off-target sites based on NGS analysis. NGS analysis revealed a significant increase in the gene editing read modification ratio in the all-in-one plasmid group compared to CT-2A WT and cells transfected with the SaCas9-KKH plasmid. In conclusion, the experimental strategy successfully targeted and edited mmu-miR-21a through the CRISPR-Cas9 system, leading to downregulation of gene expression and reduced cell proliferation rates, while demonstrating high gene editing efficiency and minimal off-target effects.
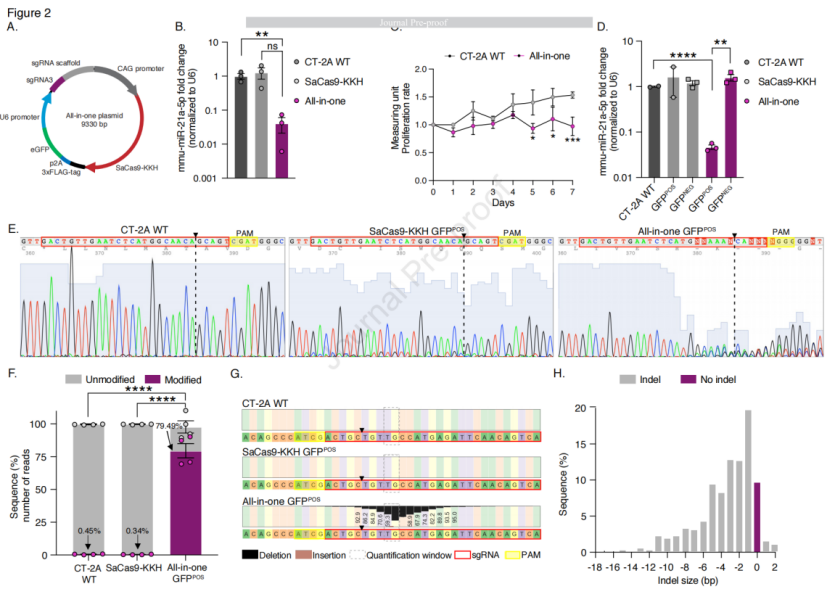
Figure 2: Single-Plasmid sgRNA3-SaCas9-KKH Reduces the Expression Level of mmu-miR-21a
The AAV8 serotype carrying the GFP gene was used to transduce GB cell lines (CT-2A and GL261) under the control of the CAG promoter. The transduction was assessed 7 days post-transduction, and imaging results showed that about 55% of cells in both GL261 and CT-2A were GFP-positive. Co-localization of GFP and Ki-67 as a percentage of total cells was approximately 50% for GL261 and 35% for CT-2A. GB cells (CT-2A-mCherry) were implanted into the brains of mice, and tumor implantation was monitored using an in vivo imaging system (IVIS). On day 7 post-implantation, AAV8-GFP or PBS (control) was intracranially injected into the mice. The mice were euthanized on day 14, and transduction efficiency was evaluated based on GFP gene expression levels. Compared to the PBS control group, mice injected with AAV8-GFP showed significantly higher GFP gene expression levels in the tumor-bearing left hemisphere, while no significant difference was observed between the groups in the non-tumor right hemisphere. Immunofluorescence (IF) analysis of the brain was performed to assess transgene expression. The results showed that AAV8-GFP exhibited the highest transduction efficiency at the tumor margin, but GFP did not co-localize with GFAP-positive astrocytes or IBA1-positive microglia in AAV8-GFP-transduced cells. However, AAV8 serotype did not only target mCherry-positive tumor cells, but GFP expression was also observed in oligodendrocytes and neurons. GFP expression levels in the liver, lungs, and spleen were low, indicating that the AAV vector was mainly sequestered by the liver, but the liver uptake of AAV8-GFP was lower, reducing the risk of liver toxicity associated with AAV-mediated delivery. Flow cytometry analysis of discrete tumor tissue from the brain showed that 13.4% of tumor cells were mCherry-positive, 3.6% were GFP-positive AAV8-transduced cells, and 3.4% were double-positive for mCherry and GFP. In conclusion, the AAV8 serotype effectively transduced GB cell lines and exhibited high transduction efficiency in the in vivo model, particularly at the tumor margin. Additionally, the biodistribution of AAV8-GFP indicated lower hepatic uptake, thereby reducing the potential risk of liver toxicity.
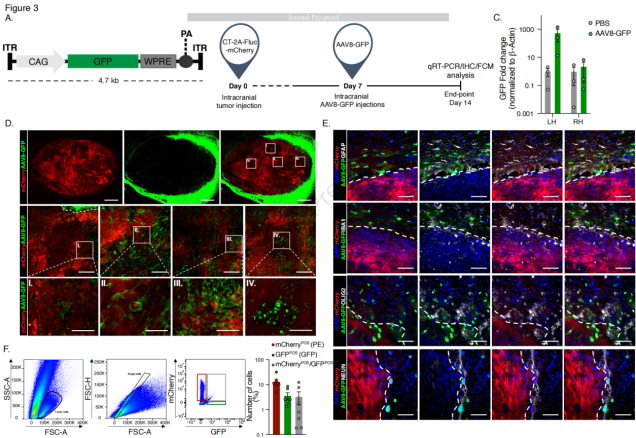
Figure 3: Transduction Profile of AAV8-GFP Vector in Mouse GB Cell Lines
To evaluate the therapeutic effect of AAV8 vector-mediated SaCas9-KKH/sgRNA gene editing of mmu-miR-21a in glioblastoma, the researchers generated a smaller 232 cassette (~4.5 kb) encoding SaCas9-KKH and sgRNA3, with these genes located between the ITRs (inverted terminal repeats) of the AAV vector. Two plasmids were constructed: one containing SaCas9-KKH and sgRNA3 (CMV-SaCas9-KKH-U6-sgRNA3, AAV8-CRISPR) and another lacking sgRNA (CMV-SaCas9-KKH-U6, AAV8-control). CT-2A cells were transfected with pre-packaged AAV8-CRISPR plasmids, and the expression level of miR-21a-5p was significantly reduced compared to the AAV8-control group. To verify the CRISPR-based editing approach, AAV vectors were co-injected with tumor cells (CT-2A-FLuc-mCherry) into the tumor site of mice via intracranial injection. Compared to AAV8-control and untreated CT-2A tumor-bearing brains, the expression level of mmu-miR-21a-5p in the brains of AAV8-CRISPR-treated mice was reduced by 180-fold. Tumor growth in AAV8-CRISPR-treated mice was suppressed between days 13 and 23, and tumors were significantly smaller on days 20 and 23. The median overall survival was significantly increased, with a 31% increase in survival time. Additionally, compared to the AAV8-control group, the expression levels of tumor suppressor genes Pten and Pdcd4 were significantly upregulated in AAV8-CRISPR-treated mice. No therapeutic effect was observed in mice treated on day 5 and day 10 post-tumor implantation, suggesting that the treatment may need to be administered within a specific time window. Sanger sequencing and NGS confirmed the insertions and deletions (indels) of the miR-21a gene sequence induced by CRISPR. Local and systemic toxicity of the AAV injections was assessed using TUNEL staining and multiple blood markers, and no local or systemic toxicity was observed due to AAV8 injection.
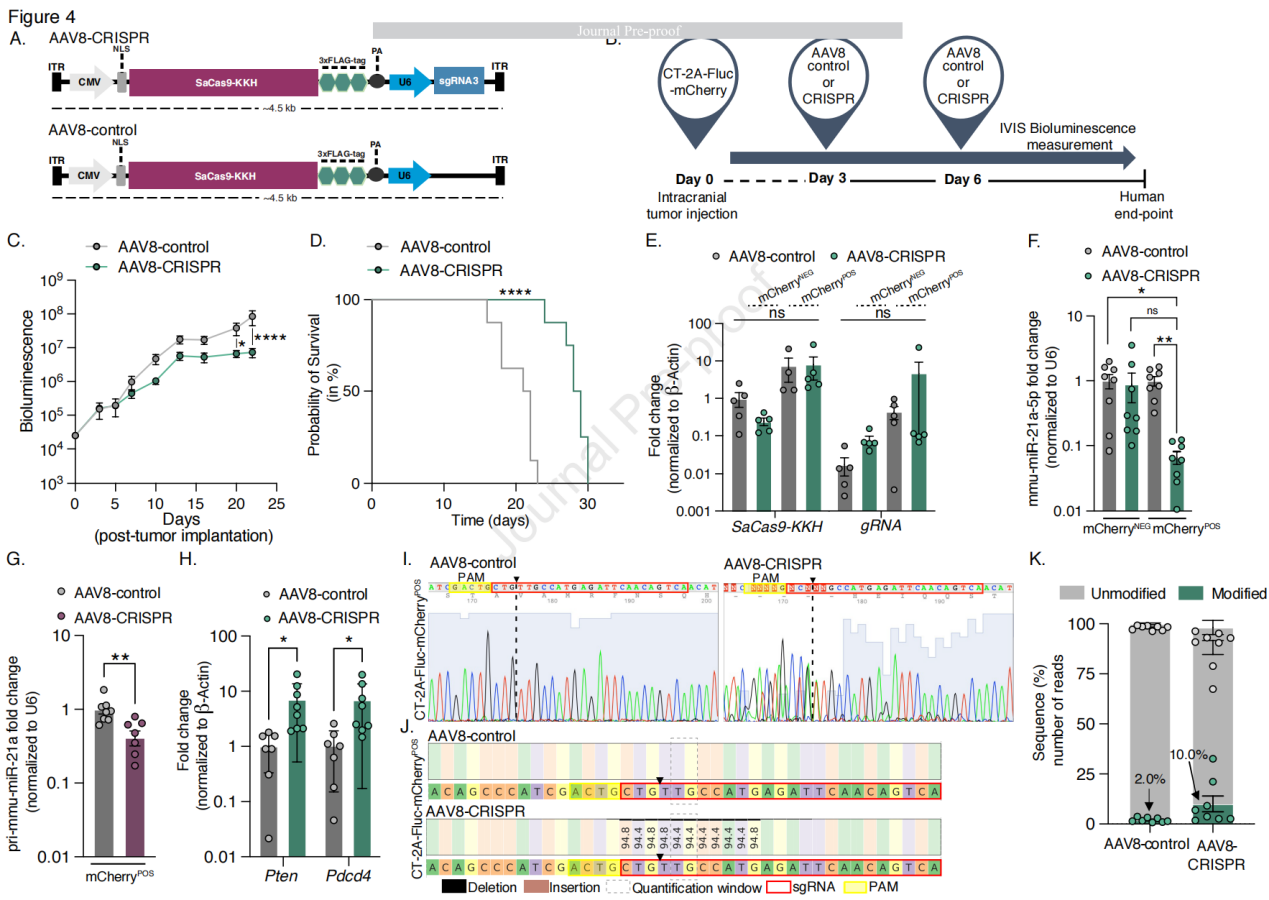
Figure 4: AAV8-CRISPR Mediates Genome Editing in Glioblastoma-Bearing Mice
The strategy described in this paper demonstrates the ability to slow tumor growth and significantly improve survival in a glioblastoma (GB) mouse model. This research not only provides a potential new therapy for glioblastoma treatment but also showcases the potential of using CRISPR-Cas9 technology for precise genome editing in cancer therapy. This has significant implications for the future development of cancer treatment strategies.
Brain Case can provide customers with a full range of vector construction, virus packaging and stable cell line construction services. If you are interested in customized services, please contact bd@ebraincase.com for details or to place an order.