- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Herpes simplex virus (HSV) is a member of the alpha-herpesvirinae subfamily of the family Herpesviridae and can specifically infect the nervous system. HSV has a wide range of animal infection hosts and can be used to analyze the neural circuits of mice, rats, non-human primates and other animals. The HSV genome is a linear double-stranded DNA molecule, about 152kb, so it can carry larger fragments of foreign genes. The virus is currently divided into type 1 and type 2 based on differences in antigenicity. The neurotropic and transneuronal propagation properties of HSV-1 make it an ideal tool for tracing neural circuits. The McIntyre-B strain of HSV-1 can spread retrogradely across neurons, while the H129 strain can spread anterogradely across neurons.
H129, as an anterograde tracer across multiple levels of synapses, has been reported to be used in various animal models to study different neural circuits. Recombinant H129 viruses expressing fluorescent proteins can be used to label output neural networks. The expression of fluorescent signals greatly facilitates the use of H129 as a tracer. HSV type 1 strain H129 (HSV-1H129) can not only mark the connections between different central brain areas, but also mark the connections between the periphery and different central brain areas.
However, for a specific brain region or cell population, the results of labeling with tracers across multiple levels cannot distinguish direct one-level output neural circuits from multi-level output neural circuits. Therefore, anterograde mono-transsynaptic tracer systems are urgently needed for neuroscience research.
HSV-ΔTK-hUbC-tdTomato is obtained by knocking out the thymidine kinase (TK) gene based on HSV-tdTomato (HSV1 strain H129 genome integrating a tdTomato gene). Due to the knockout of the TK gene, its ability to spread across synapses in vivo is limited. In the presence of a TK-expressing helper virus, HSV-ΔTK-hUbC-tdTomato can spread from the initial neurons to the postsynaptic neurons, thereby labeling the immediate downstream neurons directly innervated by a given brain area or specific type of neurons. To prevent retrograde labeling, the research team led by Professor Xu Fuqiang at the Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences, developed a rigorous and practical monosynaptic anterograde tracing recombinant virus H129 system. They embedded the anti-Her2 scFv (single-chain antibody variable) region into the gDΔ6–38 region of the H129 envelope glycoprotein D (gD) to infect neurons that only express the Her2 receptor. This achieved specificity in targeting the initial site of H129 to Her2-expressing cells, thereby eliminating the reuptake ability of the original H129 strain at axon terminals.
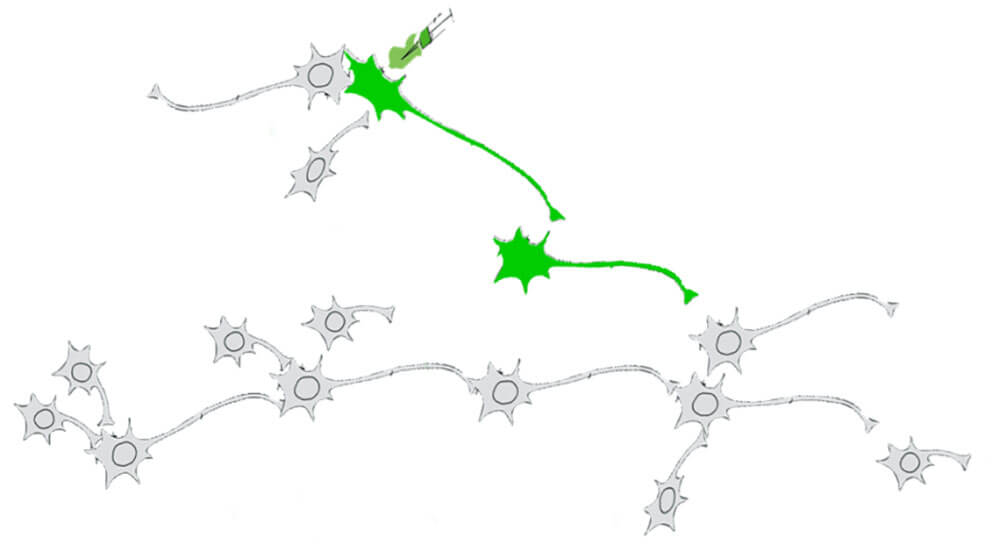
Figure 1. Diagram illustrating anterograde mono-synaptic neuronal tracing
Studying specific brain regions and neuronal types' anterograde monosynaptic output circuits (see case studies).
Example 1: Tracing neurons directly innervated by nRT-PV neurons
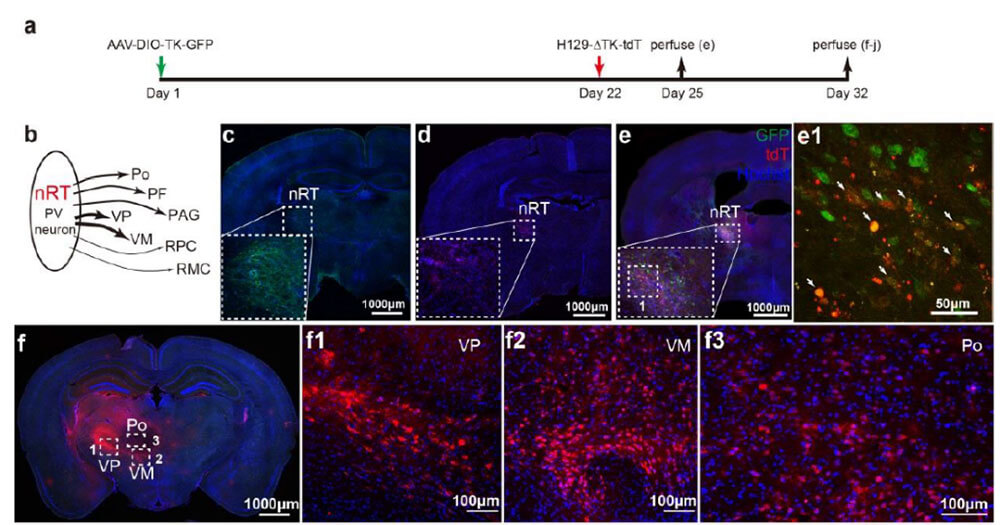
Figure 2. Tracing neurons downstream directly innervated by nRTPV neurons. (derived from Jiang Haifei’s doctoral thesis at Wuhan Institute of Virology)
Example 2: Using the Hs06 anterograde trans-monosynaptic labeling and tracing system to study the next-level neural circuits of SC
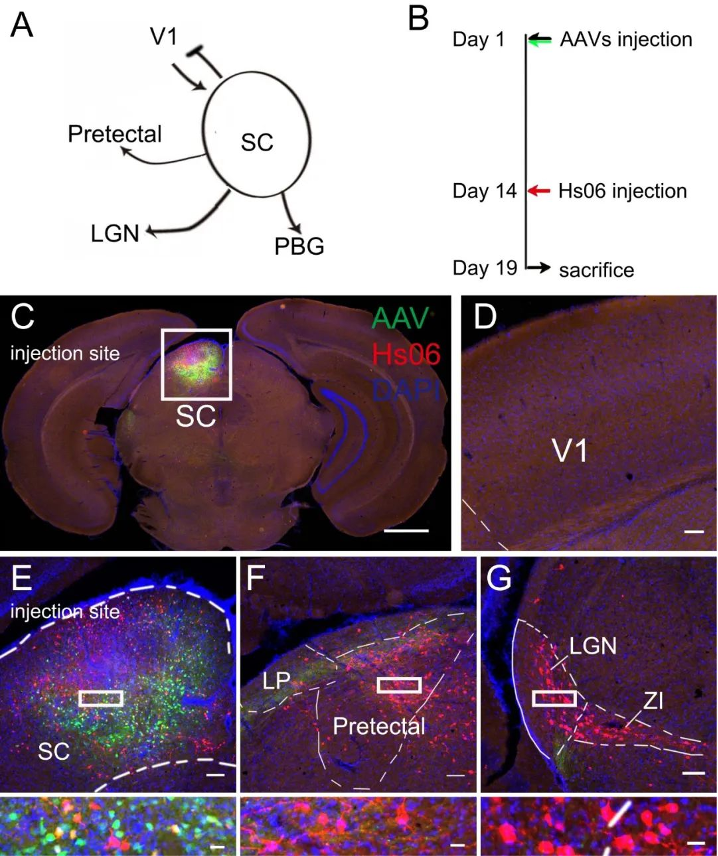
Figure 3. Rigorous anterograde trans-monosynaptic tracing of genetic defined neurons with retargeted HSV1 H129, Peng Su Fuqiang Xu. doi: https://doi.org/10.1101/2020.12.01.407312
| Product No. | Product Name | Foreign Gene Carried | Virus Color & Use |
| BC-HSC-H361 | H129ΔTK-CAG-LSL-tdTomato | tdTomato | Red, Cre-dependent, in combination with AAV-TK virus, can achieve anterograde monosynaptic tracing |
| BC-HSV-Hs06 | H129-dgD-hUbC-mCherry-P2A-scHer2::gd | mCherry | Red, Especially suitable for cell-specific anterograde trans-monosynaptic tracing output networks of the upstream starting injection site |
| BC-1245 | rAAV-hSyn-DIO-EGFP-T2A-Her2CT9 | Her2CT9 | |
| BC-1356 | rAAV-UL26.5-DIO-cmgD | cmgD |
| Labeling Type | Synaptic Specificity | Common Tools |
|---|---|---|
| Retrograde Tracing | Non-synaptic | AAV2/Re, AAV2/11, RV-ΔG-N2cG, CTB |
| Retrograde Monosynaptic Tracing | Monosynaptic | RV-EnvA-ΔG-XFP |
| Retrograde Mutisynaptic Tracing | Mutisynaptic | PRV-XFP, PRV-△TK-DIO-XFP (Cre-dependent) |
| Anterograde Tracing | Non-synaptic | AAV2/2, AAV2/8, AAV2/9 |
| Anterograde Monosynaptic Tracing | Monosynaptic | AAV2/1, AAV2/9-mWGA, Hs06, H361 |
| Anterograde Mutisynaptic Tracing | Mutisynaptic | HSV, VSV |
| Deliverables (for all services above): Full project report and raw imaging data | ||