- E-mail:BD@ebraincase.com
- Tel:+8618971215294
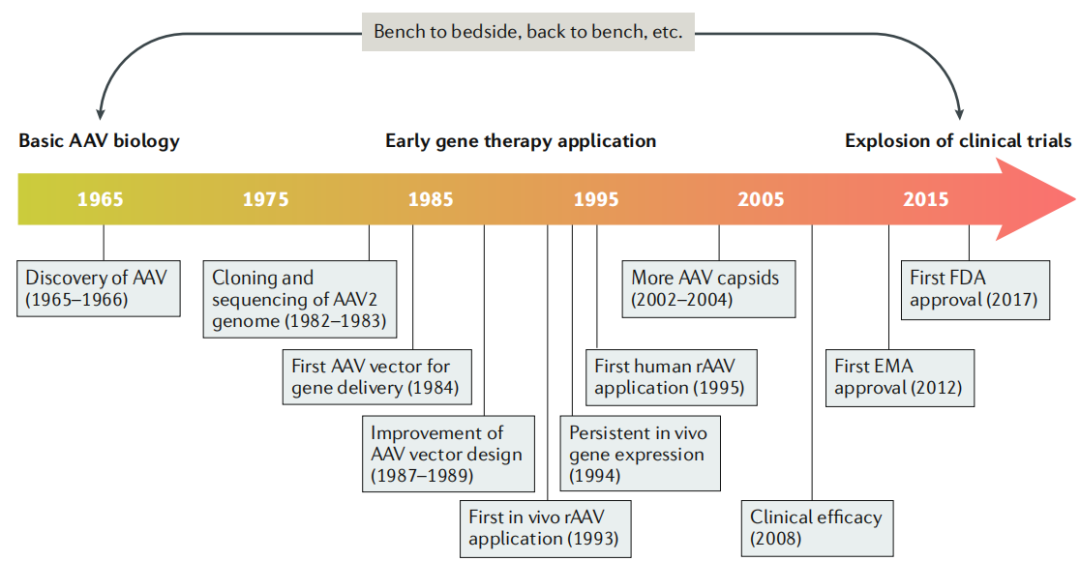
AAV Viral Properties:
AAV belongs to the Parvoviridae family, Dependoparvovirus genus. Its life cycle depends on helper viruses, such as adenoviruses (AdV). The virus is composed of an ~26 nm icosahedral protein capsid and a ~4.7 kb single-stranded DNA genome. The capsid comprises three subunits—VP1, VP2, and VP3—that work together to protect the viral genome. The ITRs at both ends of the genome play essential roles in replication and packaging. Within the AAV genome, the rep gene encodes four replication proteins involved in genome replication; the cap gene encodes the capsid subunits, determining its structure and composition; and there is also a gene encoding the assembly-activating protein (AAP), which is crucial for capsid assembly.
rAAV Vector Characteristics:
rAAV vectors retain the capsid sequence and structure of wild-type AAV but have a modified genome. All viral coding sequences are removed and replaced with a therapeutic gene expression cassette, leaving only the ITRs intact. As a result, rAAV has a limited packaging capacity—less than 5.0 kb—necessitating careful consideration of the therapeutic gene's size and the regulatory elements included during vector design.
rAAV Transduction Process:
rAAV enters cells by binding to surface receptors and undergoing clathrin-mediated endocytosis. Once inside, it experiences pH-dependent conformational changes, is transported via the cytoskeletal network, and enters the nucleus through nuclear pores. Inside the nucleus, the released single-stranded genome must be converted into double-stranded DNA before transcription can occur. This conversion can happen through second-strand synthesis or strand annealing. The genome may then circularize into episomal DNA, enabling gene expression.
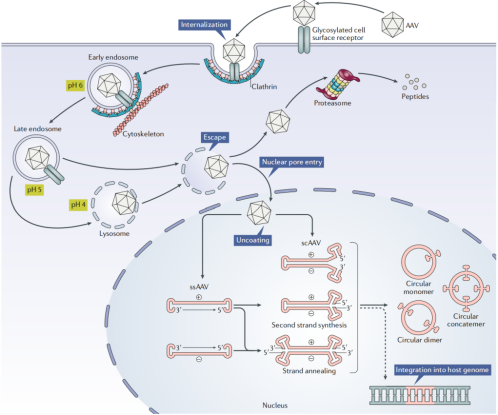
Figure 2 Schematic Diagram of the rAAV Transduction Pathway
Capsid Development Strategies:
To achieve novel properties, researchers have employed various methods to develop new AAV capsids. These include:
Natural discovery (e.g., AAV9 isolated from human liver tissue, capable of crossing the blood-brain barrier),
Rational design (e.g., engineering AAV2 to alter its tropism and immunogenicity),
Directed evolution (e.g., the CREATE method identified AAV-PHP.B, which can cross the blood-brain barrier),
Computational design (e.g., Anc80, predicted in silico, shows strong efficacy across multiple tissues).
Key Points in Genome Design:
The first goal is to control gene expression. This is achieved by selecting suitable promoters for precise expression in specific cells or tissues and optimizing codons to improve expression efficiency and specificity. To accommodate large genes, strategies include designing shortened therapeutic genes (e.g., mini-dystrophin for Duchenne muscular dystrophy) or co-delivering two AAV vectors. To enhance expression durability, techniques such as genome editing, insertion of scaffold/matrix attachment region (S/MAR) sequences, and other regulatory elements can be used to promote genome integration and sustained expression.
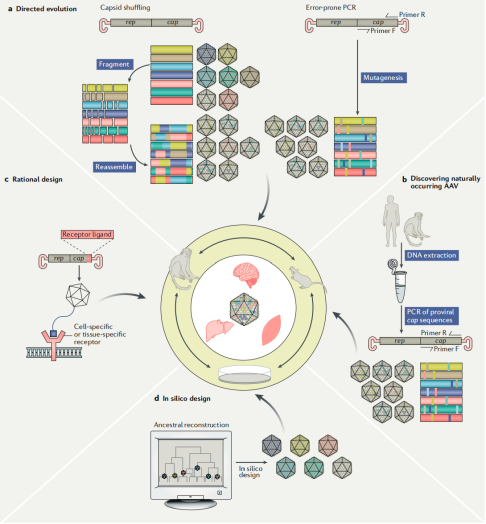
Figure 3 Infographic of the Four Main Approaches to Capsid Discovery and Engineering
Current Clinical Status:
As of November 13, 2018, a total of 145 interventional clinical trials involving recombinant AAV (rAAV) have been registered on ClinicalTrials.gov. AAV1 (Glybera) and AAV2 (Luxturna) have received regulatory approval for commercial use. Commonly used capsid serotypes in clinical trials include AAV2, AAV8, and AAV9.
Therapeutic Strategy Categories:
Gene replacement is primarily used to treat recessive monogenic disorders (e.g., Glybera for lipoprotein lipase deficiency, Luxturna for inherited retinal dystrophy).
Gene silencing targets monogenic diseases caused by toxic gain-of-function mutations (e.g., Huntington’s disease), though most such approaches are still in preclinical development.
Gene addition can be applied to complex genetic or acquired diseases (e.g., delivering recombinant antibodies via rAAV to treat HIV infection).
Gene editing uses programmable nucleases to correct disease-causing mutations (e.g., in vivo rAAV-based gene editing for lysosomal storage disorders).
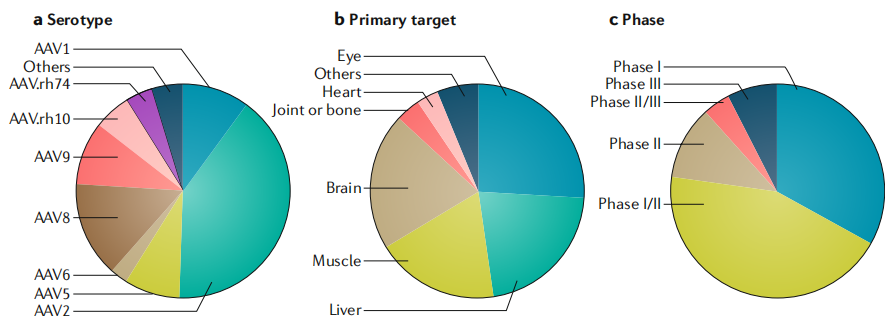
Figure 4 Overview of Clinical Trials Involving rAAV-Mediated Gene Therapy
Production and Cost Issues:
AAV gene therapy drugs are extremely expensive—for example, Glybera once cost $1.2 million per patient, and Luxturna is priced at $425,000 per eye. The primary reasons are the difficulty and high cost of large-scale manufacturing. Current production systems have relatively low efficiency, and the cost of clinical trials remains high.
Quality Control Challenges:
There is a wide variation in the ratio of empty to full capsids across different preparations (empty capsids can account for 20%–98%). Vector potency is influenced by many factors, with poor reproducibility in in vivo models, though in vitro surrogate assays are rapidly developing. In terms of DNA content, impurities may pose risks, and there is currently no unified standard for assessing DNA levels. Regarding capsid properties, novel capsids must be accurately assessed for stability and heterogeneity.
Immunity-Related Barriers:
Neutralizing antibodies (NAbs) against AAV capsids are common in humans and can block gene delivery. Administration of AAV vectors also triggers humoral and cellular immune responses, reducing therapeutic efficacy. Furthermore, AAV vectors can activate innate immunity, necessitating strategies to mitigate immune reactions.
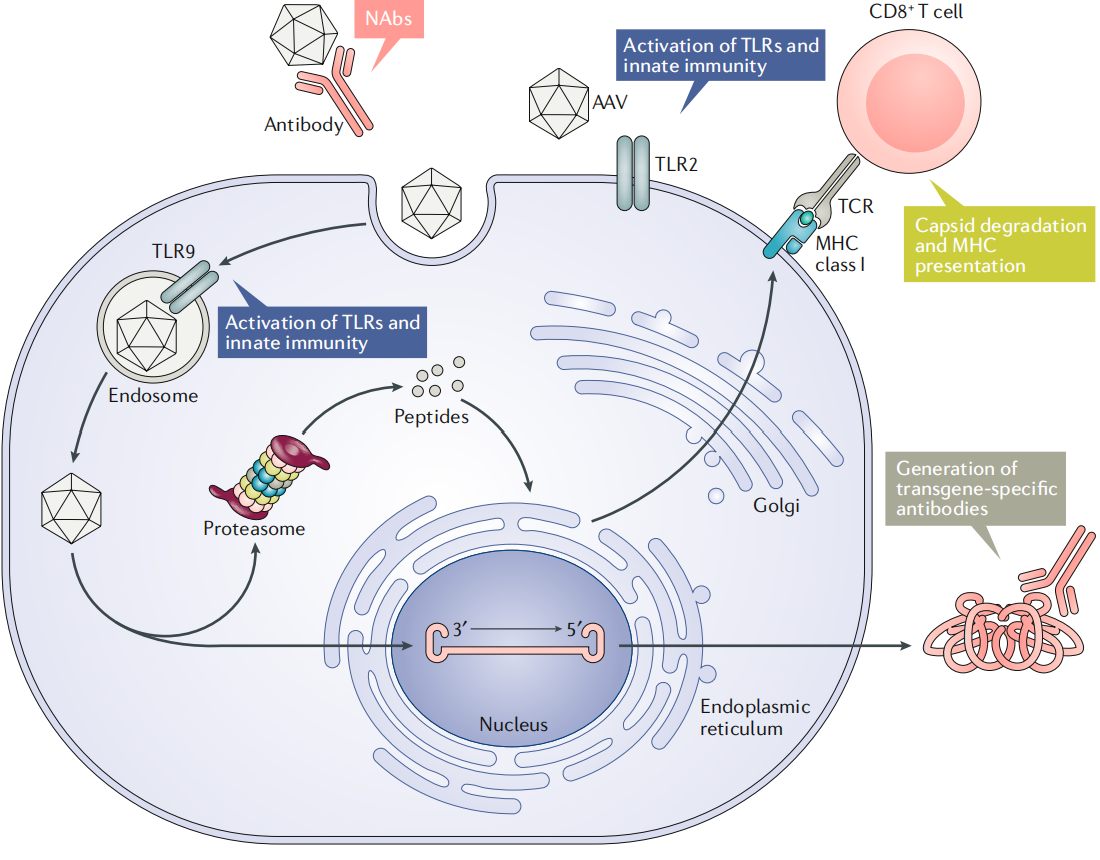
Figure 5 Immune Barriers to Successful rAAV Gene Delivery
AAV vectors offer several advantages. First, AAV is currently considered non-pathogenic to humans, providing a favorable safety profile. Second, recombinant AAVs have had all viral coding sequences removed, retaining only the ITRs, resulting in low immunogenicity and cytotoxicity. Third, AAV vectors have a broad host range and can infect various cell types and tissues. Fourth, they enable long-term and stable gene expression, as the viral genome can persist in cells in the form of episomal DNA.
Immune barriers associated with AAV vectors significantly affect gene therapy outcomes. Pre-existing neutralizing antibodies (NAbs) in the bloodstream can effectively block rAAV delivery, especially via intravenous injection. Following administration, the AAV capsid can trigger strong humoral immune responses that generate NAbs, making re-administration largely ineffective. Capsids may also activate cytotoxic T lymphocytes (CTLs), potentially eliminating transduced cells and leading to loss of transgene expression. Additionally, the transgene product may induce adaptive immune responses, resulting in the formation of product-specific antibodies and CTLs, which further compromise therapeutic efficacy. Moreover, AAV vectors can activate innate immunity, promoting the release of inflammatory cytokines and amplifying the adaptive immune response.
To address production and cost challenges, researchers are developing more efficient manufacturing systems by optimizing cell culture conditions and transfection methods to increase vector yield and reduce cost. Alternative platforms, such as cell-free production systems, are also being explored.
For quality control, efforts focus on developing more accurate analytical methods to evaluate the ratio of empty to full capsids, vector potency, DNA content, and capsid properties, as well as establishing unified quality standards.
To overcome immune barriers, strategies include engineering AAV capsids to eliminate NAb binding sites and reduce immunogenicity, and exploring immune modulation approaches such as inducing immune tolerance or suppressing immune responses to enhance therapeutic outcomes.
Original article link: https://www.nature.com/articles/s41573-019-0012-9
The Brain Case team is dedicated to advancing AI technologies and platforms by leveraging our proprietary high-yield AAV production system, featuring large capacity and a high-precision AAV mutagenesis engineering and screening platform. With a strong focus on cost reduction and efficiency enhancement, we aim to develop AAV vectors with improved targeting efficiency and precision for specific tissues or cell types, supporting applications in gene therapy, cell therapy, and related biomedical fields.
If you're looking to obtain an AAV serotype that specifically infects target cells, please contact us at BD@ebraincase.com