- E-mail:BD@ebraincase.com
- Tel:+8618971215294
In the research of neurodegenerative diseases, microglia, as resident immune cells of the central nervous system (CNS), have gradually become a focal point for scientists' in-depth exploration. These cells play an indispensable role in various physiological processes, including synaptic pruning, injury repair, homeostasis maintenance, phagocytosis, and supporting communication between other glial cells and other cell types. Notably, microglia are closely linked to the occurrence and development of various neurodegenerative diseases.
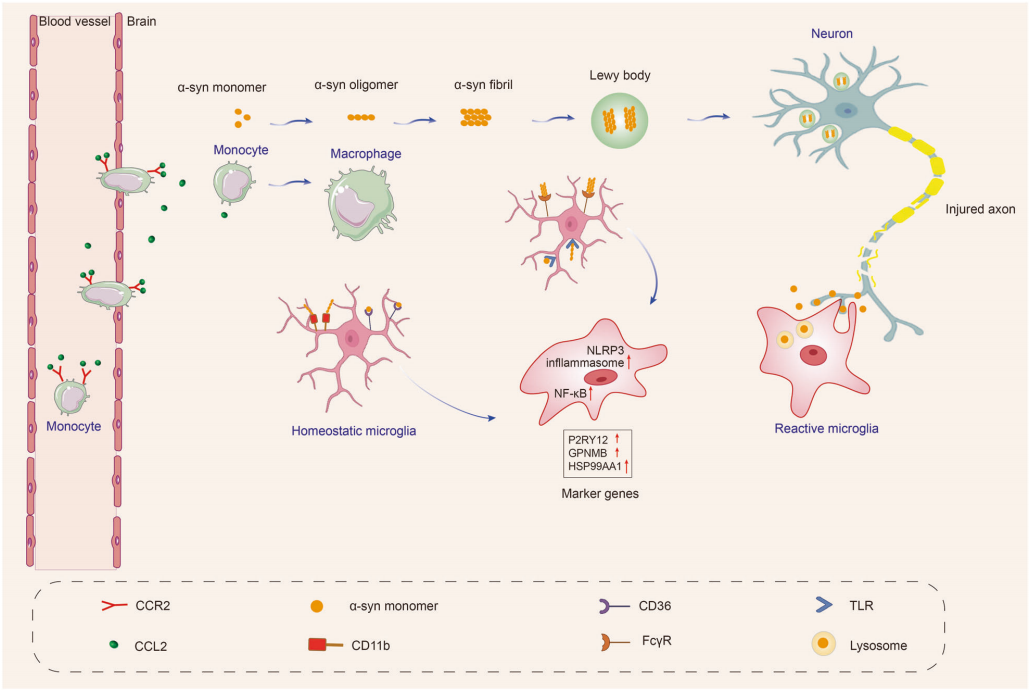
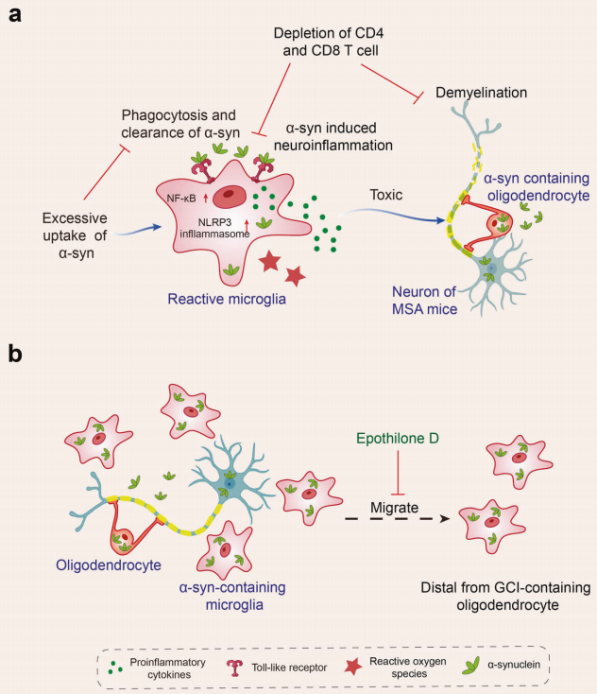
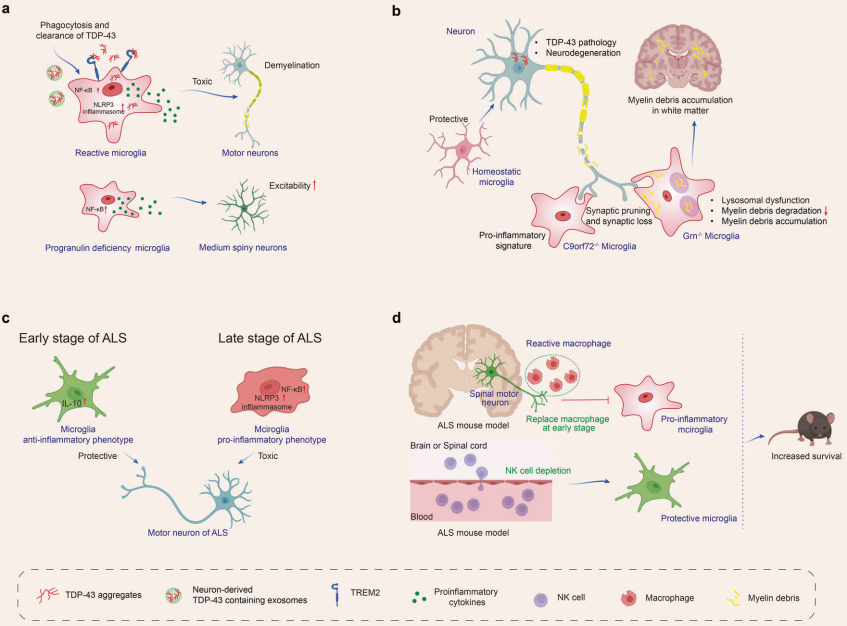
When the central nervous system is damaged or affected by disease, microglia exhibit a complex response, often referred to as "activation." In early studies of microglia, scientists primarily detected the activation state of these cells through morphological observations, specifically by noting their transformation from a branched phenotype in healthy brains to an amoeboid appearance in diseased brains. However, as research has progressed, scientists have found that the activation process of microglia is far more diverse and dynamic than previously anticipated, as reflected in both omics characteristics and functional outcomes. This indicates that microglia present different response patterns in various diseases. Currently, research has observed microglial activation in multiple neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), multiple system atrophy (MSA), amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD), progressive supranuclear palsy (PSP), dementia with Lewy bodies (DLB), and Huntington's disease (HD).
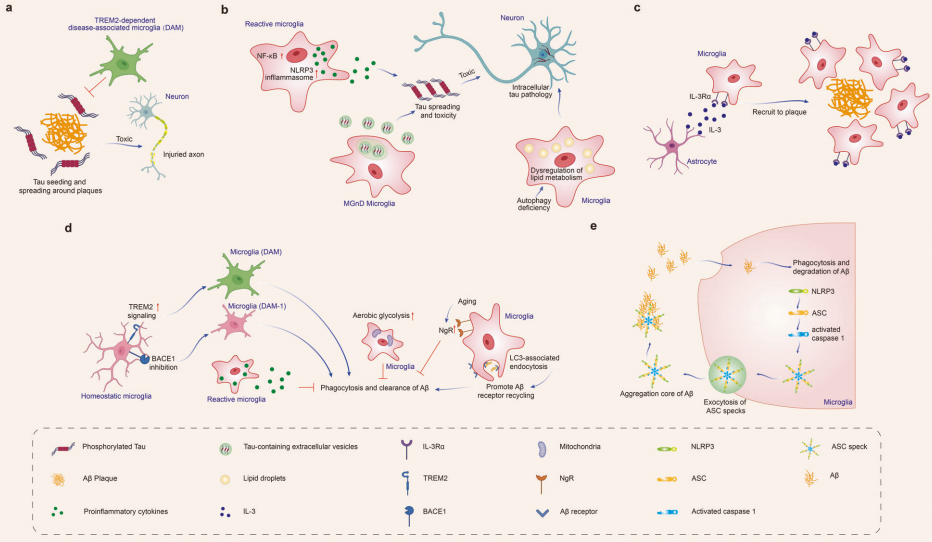
AD is one of the most well-known neurodegenerative diseases, characterized primarily by the accumulation of beta-amyloid (Aβ) plaques and the abnormal aggregation of tau protein, leading to neurofibrillary tangles within neurons. Imaging studies have found that the activation of microglia in AD is associated with tau and amyloid proteins. These activated microglia have been observed to accumulate near Aβ plaques in various regions of the brains of AD mice and in humans post-mortem. In AD mouse models, a subset of microglia, referred to as disease-associated microglia (DAM), was first identified, which participates in the clearance of Aβ.
Research indicates that microglia play a double-edged role in the progression of AD. On one hand, they can phagocytize and clear Aβ and tau proteins, limiting the spread of these pathological substances and potentially offering protective effects in the early stages of the disease. On the other hand, prolonged stimulation of microglia by pathological deposits may lead to dysfunction, promoting neuroinflammation, accelerating the spread of Aβ and tau, and resulting in neurodegeneration.
A substantial body of evidence shows that microglia affect Aβ and tau proteins in various ways:
Based on the in-depth understanding of the complex relationship between neurodegenerative diseases and microglia, researchers are actively exploring a range of innovative therapeutic strategies aimed at promoting disease treatment by modulating microglial function. The principles and effects of these strategies have been preliminarily validated in cell experiments, animal models, and clinical trials.
Modulating neuroinflammation is one of the most widely used therapeutic targets, focusing on intervening in the activation state of microglia through drugs or other means to reduce the release of harmful inflammatory factors and thereby alleviate neuronal damage. Research has found that using the NLRP3-specific inhibitor OLT1177 to inhibit the NLRP3 inflammasome can rescue cognitive impairments in AD mouse models. Similarly, the NLRP3 inhibitor MCC950 has been shown to suppress inflammasome activation, α-synuclein aggregation, and dopaminergic degeneration in the substantia nigra of PD mouse models, improving motor function.
Microglia release exosomes containing tau, and microglial activation can facilitate tau propagation. Inhibiting the synthesis and secretion of microglial exosomes can help reduce tau proliferation. Studies have shown that early pharmacological blockade of P2RX7 in tau mice significantly impairs microglial exosome secretion, reducing tau accumulation in the brain and thereby improving working and learning memory.
Microglial phagocytosis requires substantial energy. When microglia switch their metabolism from oxidative phosphorylation to aerobic glycolysis, sustained aerobic glycolysis can impair their immune function. Research indicates that modulating microglial metabolism can enhance their phagocytic capacity for Aβ. For instance, treatment with sodium rutin (NaR) shifts microglial metabolism from anaerobic glycolysis to mitochondrial oxidative phosphorylation, providing sufficient ATP for Aβ clearance. Additionally, NaR promotes the clearance of Aβ by increasing the expression levels of phagocytosis-related receptors in microglia.
The brain microenvironment can regulate the phenotypic transformation of microglia, promoting their positive functions. In AD, a ROS-responsive polymer micelle system can accumulate in lesioned areas by mimicking Aβ transport pathways. These micelles normalize the oxidative and inflammatory microenvironment, promoting microglial regeneration. In PD, vitamin D protects dopaminergic neurons from inflammation and oxidative stress by inhibiting microglial activation and promoting M2 polarization, increasing the expression of M2 microglial markers such as CD163, CD204, and CD206.
Activating the expression of key genes such as TREM2 is also considered to enhance the clearance capacity of microglia, promoting their effective clearance of pathological proteins like Aβ and tau. Studies have shown that enhancing TREM2 signaling through TREM2 agonist antibodies such as 4D9, AL002c, or tetravalent TREM2, or using a gene delivery system targeting microglia to upregulate TREM2 levels in the brain, can enhance Aβ clearance and alleviate neuroinflammation.
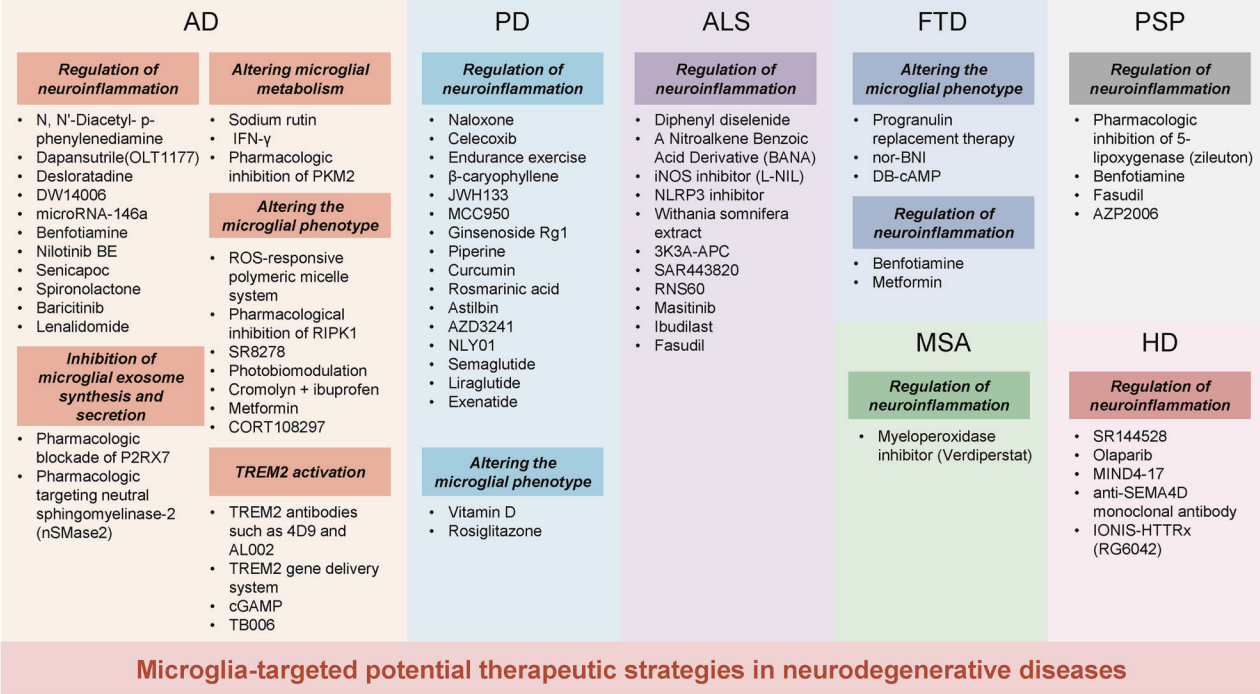
Figure 5: Potential microglial-targeted interventions and therapies for neurodegenerative diseases.
In summary, the role of microglia in neurodegenerative diseases is multifaceted and complex. Through in-depth research and exploration of therapeutic strategies, new insights have been provided for the treatment of neurodegenerative diseases, laying a solid foundation for the future development of more precise and effective therapies.
Brain Case has launched an innovative AAV11 serotype vector, model BC-ZA0182: rAAV-mIBA1-EGFP-WPRE-4×miR-9.T, specifically designed for the efficient and selective transduction of microglia. Furthermore, this vector is flexibly designed to allow for the overexpression or interference of specific genes, or to integrate techniques such as optogenetics, chemogenetics, and calcium signaling recording, thereby providing a powerful genetic manipulation toolset for exploring the functions, mechanisms, and roles of microglia in neurological diseases.
References
Gao C, Jiang J, Tan Y, Chen S. Microglia in neurodegenerative diseases: mechanism and potential therapeutic targets. Signal Transduct Target Ther. 2023 Sep 22;8(1):359. doi: 10.1038/s41392-023-01588-0. PMID: 37735487; PMCID: PMC10514343.