- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Key goals in contemporary biomedical research include elucidating the molecular basis of cardiovascular diseases and developing new methods for treatment and prevention. To accelerate basic scientific research in animal models and realize the potential for direct clinical applications, the development of efficient in vivo gene transfer techniques is crucial. However, progress in human gene therapy is limited by the constraints of existing gene delivery systems. An ideal vector for cardiac gene therapy should efficiently and specifically deliver genes to cardiomyocytes, provide long-term gene expression, elicit no immune response, pose minimal risk to the host, and allow for administration without the need for complex surgical procedures.
In the past, to focus virus-mediated gene delivery on the heart, techniques such as direct injection into the myocardium and complex coronary perfusion methods requiring thoracotomy were primarily used. Conventional AAV serotypes (AAV2), commonly used in previous AAV studies, have limitations, including relatively low transduction rates and a delay of up to six weeks for full expression. Recent studies have shown that in addition to the AAV2 serotype, AAV1, AAV6, AAV8, and AAV9 can also effectively cross the endothelial barrier and efficiently transduce various organs. The AAV9 capsid has been reported to exhibit a stable preference for cardiac tissue in vivo, both in neonatal and adult mice.
AAV serotypes AAV-1, AAV-6, AAV-8, and AAV-9 can effectively cross the endothelial barrier. In in vivo cardiac studies, AAV9 and AAV8 serotypes show higher infection efficiency in the heart compared to other serotypes, with AAV9 being the most commonly used.
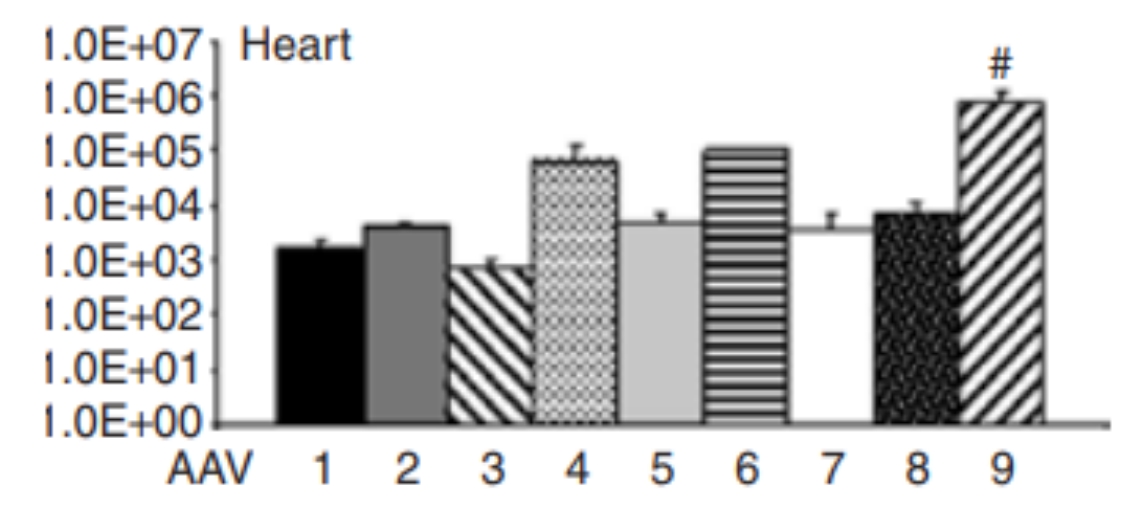
Figure 1 Expression Profiles of Different Serotypes in the Heart
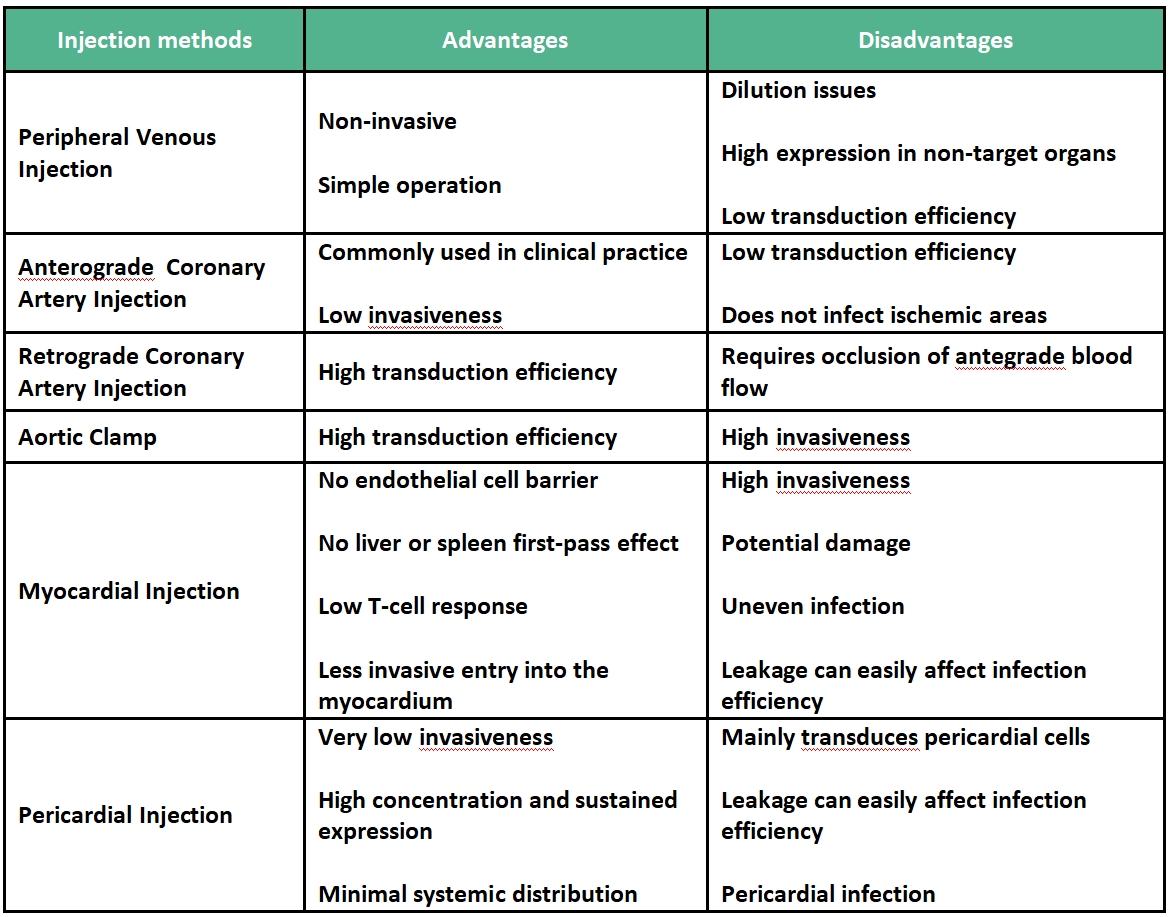
There are various methods for gene delivery targeting the heart, as shown in the figure below. The choice of the optimal approach largely depends on whether the target area is a specific region of the heart (e.g., the area of myocardial infarction) or the entire heart. For infecting the entire heart, options include intravenous or arterial injection, or multiple intramyocardial injections. If targeting a specific site in the heart, direct injection can be performed from the outside of the heart or onto the endocardial surface.
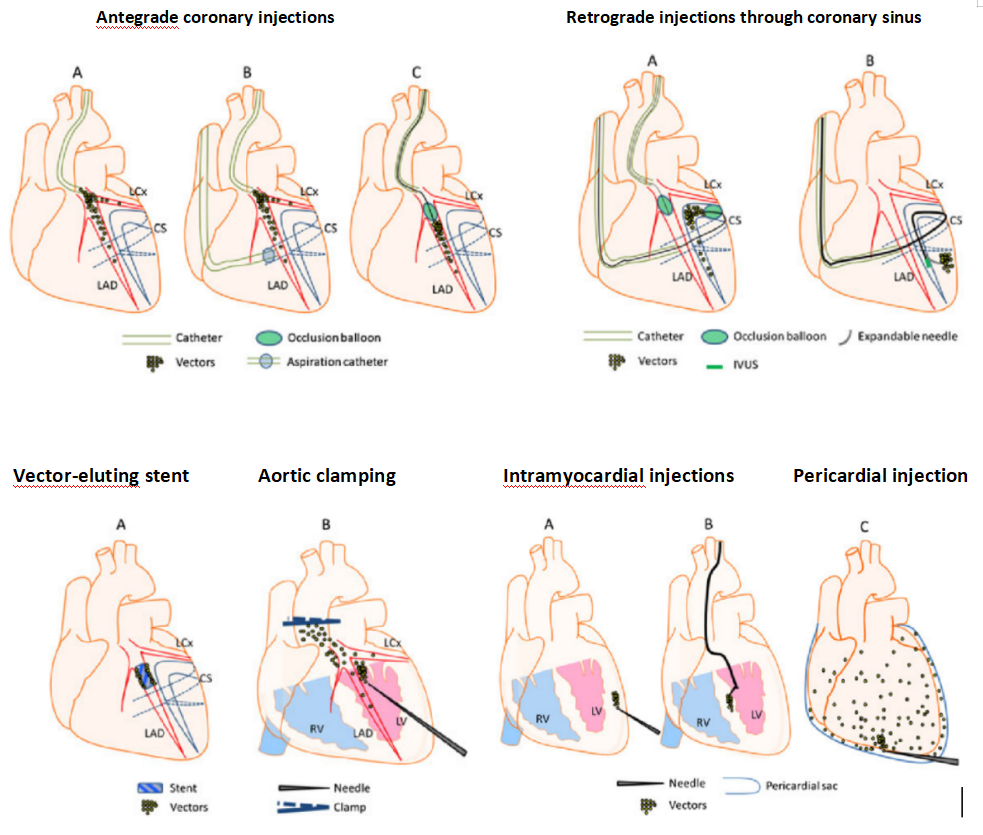
Figure 2 Schematic Diagram of Common Cardiac Injection Methods
Example 1
Comparison of the Specific Infection of Cardiomyocytes and Gene Transfer Efficiency of Five AAV Serotypes
Vectors: AAV1, AAV2, AAV6, AAV8, AAV9
Promoters: CMV, cTNT
Experimental Animals: C57 mice
Injection Protocol: Jugular vein injection, 1×10^11 vg per mouse, expression for 4 weeks.
Experimental Results: Fluorescence microscopy was used to observe frozen heart sections from mice treated with AAV vectors packaged in AAV capsid serotypes 1, 2, 6, 8, and 9. AAV-cTnT-eGFP was packaged in AAV capsids from specified serotypes (AAV1, AAV2, AAV6, AAV8, or AAV9). One-week-old mice were injected with the specified dose of viral genomes via the jugular vein. Four weeks after administration, 6 μm frozen heart sections were prepared and analyzed with a fluorescence microscope. The results showed that AAV9 transduced cardiomyocytes more efficiently than other serotypes.
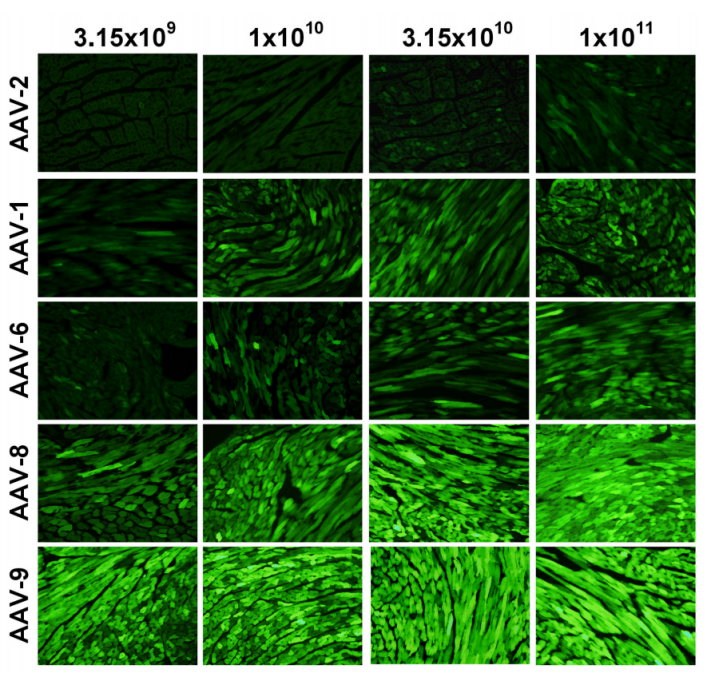
Figure 3 AAV Vector Provides Homogeneous Transduction of Cardiomyocytes Across the Entire Ventricular Myocardium
Example 2
Non-Invasive Optogenetic Cardiac Regulation
Vector: AAV9
Promoter: mTNT
Experimental Animals: C57 mice
Injection Protocol: Retro-orbital vein injection, 2×10^11 vg per mouse, expression for 3 weeks
Experimental Results: To achieve optogenetic regulation of cardiac rhythm, AAV9-mTNT-ChRmine-P2A-oScarlet was administered via retro-orbital vein injection to enable specific expression of ChRmine in cardiomyocytes. When 589 nm light pulses were delivered through the intact skin covering the chest of anesthetized mice, heart rates of up to 900 bpm were induced, with immediate recovery of sinus rhythm after cessation of the light.
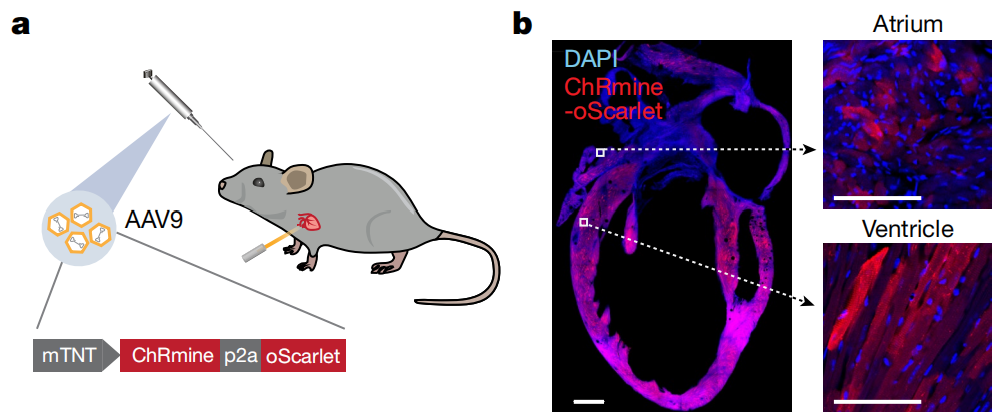
Example 3
Cardiac-Specific Overexpression of Caveolin-1 in Rats with Ischemic Cardiomyopathy
Vector: AAV9
Promoter: cTNT
Experimental Animals: Rats
Injection Protocol: Myocardial injection, expression for 4 weeks
Experimental Results: To achieve cardiac-specific overexpression of Cav1, AAV9-cTNT-Cav1-EGFP was delivered via intramyocardial injection in rats. The virus mediated Cav1 expression under the control of the cardiac troponin T (cTnT) promoter. Additionally, the Sham control group received intramyocardial injection of the control virus AAV9-cTNT-EGFP. The results showed that cardiac-specific overexpression of Caveolin-1 in the ischemic cardiomyopathy rat model improved arrhythmia and cardiac remodeling.
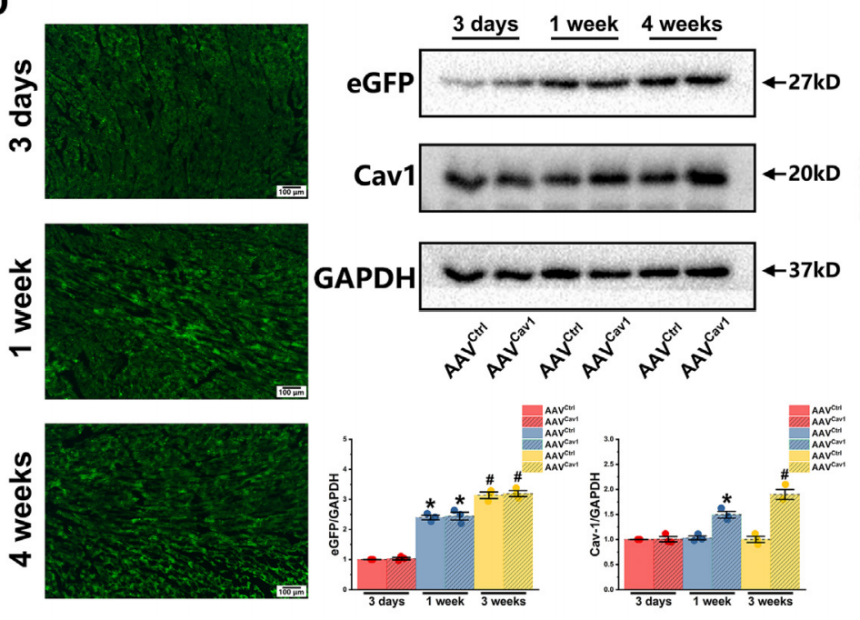
Example 4
Cardiac-Specific Overexpression of Cb1r in Mice
Vector: AAV9
Experimental Animals: WT C57 mice
Injection Protocol: Injection into 3-5 sites in the left ventricle of the heart, 2×10^11 vg/ per mouse, expression for 3 weeks
Experimental Results: An AAV9-mediated Cb1r overexpression virus, AAV9-CB1R, was constructed and injected in situ into the mouse heart. The results showed that overexpression of Cb1r in WT mice induced the expression of cardiac pyroptosis factors and pro-fibrotic factors, significantly inhibited left ventricular function, and promoted an increase in heart weight. However, in Gsdmd−/− mice, treatment with AAV9-CB1R alone or in combination with Olz (Olanzapine) no longer induced the above effects.
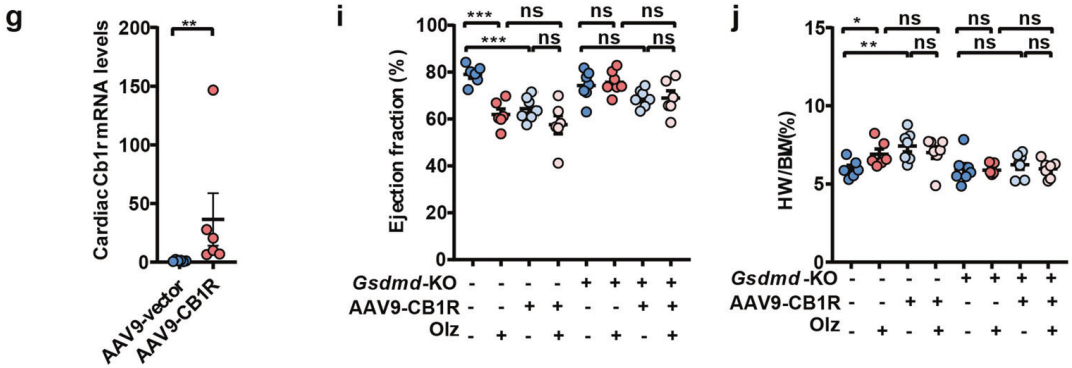
Brain Case can provide and customize related products. If needed, you can consult BD@ebraincase.com.