- E-mail:BD@ebraincase.com
- Tel:+8618971215294
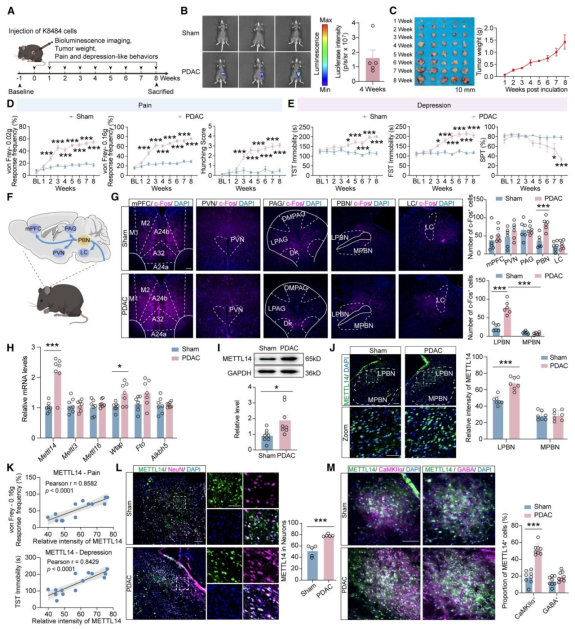
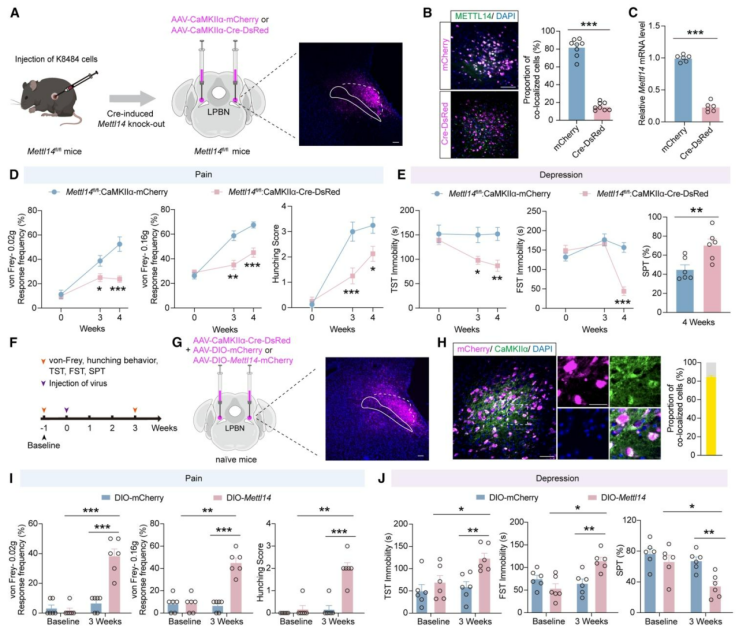
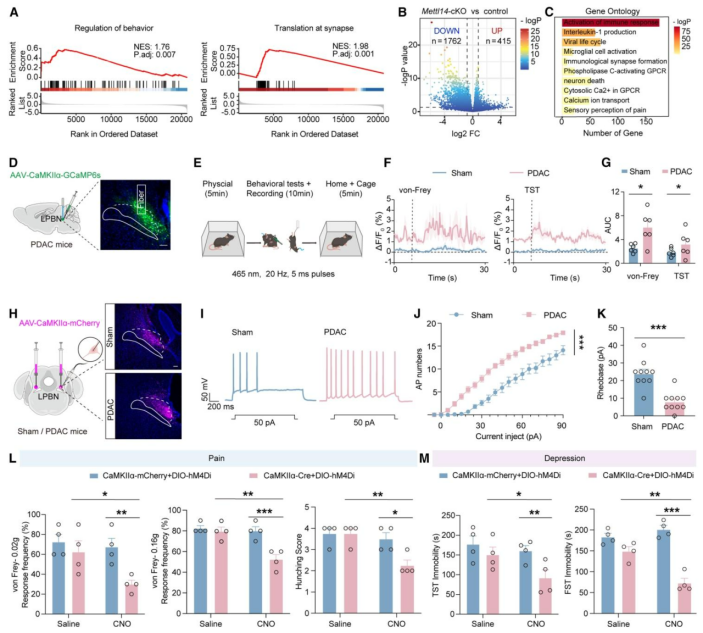
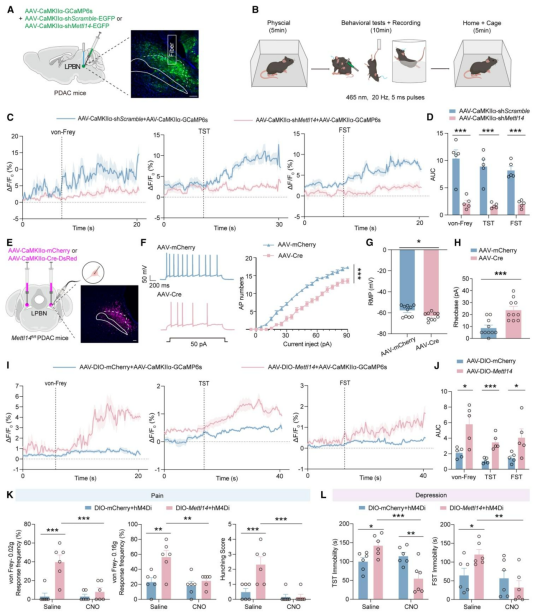
| Product Categories | Number | Product Name |
|---|---|---|
| Fluorescent Proteins | BC-0015 | rAAV-EF1α-DIO-EGFP |
| BC-0016 | rAAV-EF1α-DIO-mCherry | |
| BC-0027 | rAAV-CaMKIIα-EGFP | |
| BC-0028 | rAAV-CaMKIIα-mCherry | |
| Recombinases | BC-0164 | rAAV-CaMKIIα-Cre |
| BC-0159 | rAAV-hSyn-SV40NLS-Cre | |
| BC-1468 | AAV-CaMKIIα-Cre-P2A-DsRed2 | |
| Chemogenetic | BC-0155 | rAAV-EF1α-DIO-hM4D(Gi)-mCherry |
| Optogenetic | BC-0126 | rAAV-EFα-DIO-eNpHR3.0-mCherry |
| Calcium Indicator | BC-0081 | rAAV-CaMKIIα-GCaMP6s |