- E-mail:BD@ebraincase.com
- Tel:+8618971215294
In previous whole-cell patch-clamp recordings, the authors observed that some MM neurons exhibited two distinct patterns of spontaneous firing: tonic and bursting. To further investigate this phenomenon, they switched to cell-attached recordings and found that MMCB neurons had a lower rate of spontaneous activity compared to MMPV neurons, but a higher incidence of burst firing. Some MM neurons were capable of switching between tonic and bursting modes.
N — methyl-D-aspartate receptors (NMDARs) and T-type voltage-sensitive calcium channels (T-VSCCs) have been reported to play key roles in neuronal burst firing across various brain regions. The authors further examined this and found that activation of NMDARs promoted the transition of most tonic-firing neurons into a bursting mode. Notably, MMCB neurons generated more action potentials per burst with longer inter-burst intervals. Additionally, MMCB neurons exhibited a higher incidence of post-hyperpolarization rebound firing mediated by T-VSCCs, consistent with their bursting characteristics.
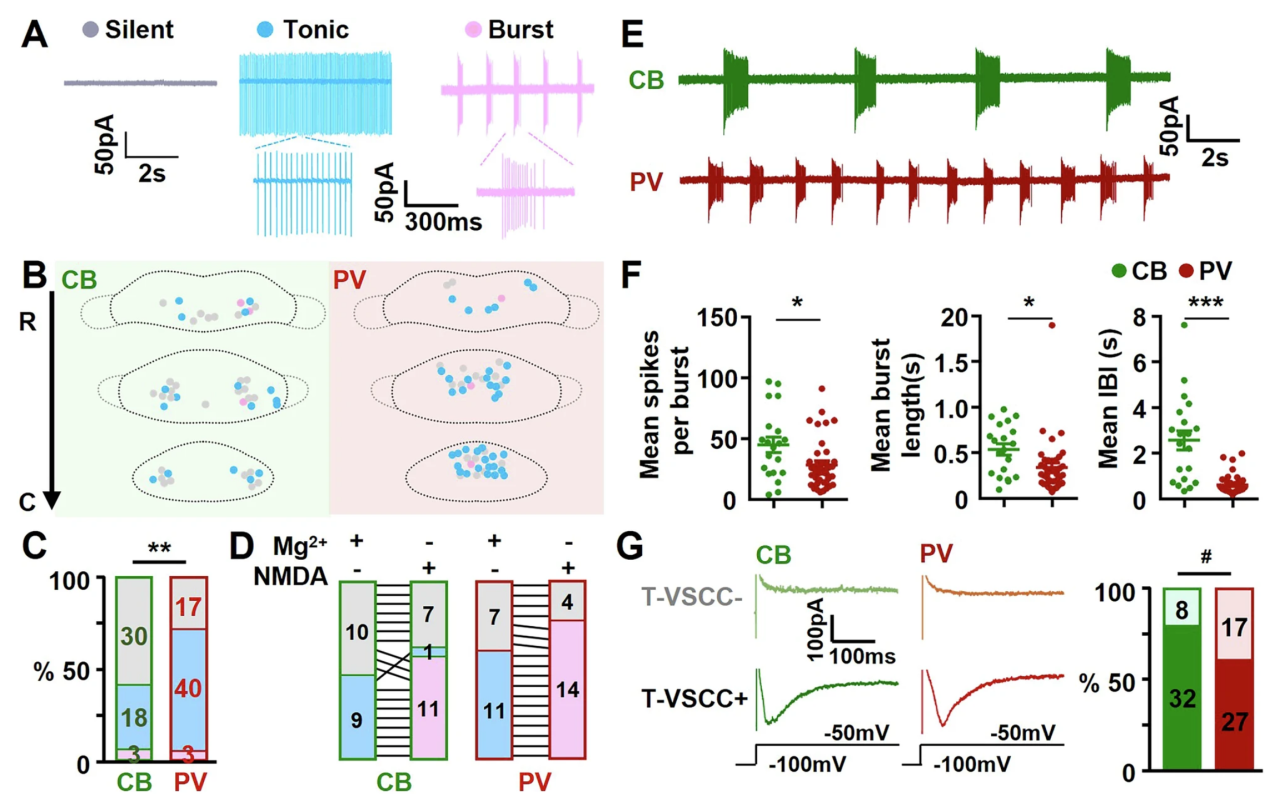
Figure 3. Differences in spontaneous firing between MMCB and MMPV neurons.
To visualize the output projections of MMCB and MMPV neurons, the researchers injected AAV-DIO-ChR2-RFP and AAV-fDIO-ChR2-GFP into the MM region of CBcre::PVFlp mice. The results showed that the axon terminals of MMCB and MMPV neurons exhibited minimal overlap in downstream brain regions such as the anterior thalamus (AT), ventral tegmental nucleus (VTg), and retrotegmental pontine nucleus (RtTgP). This indicates that MMCB and MMPV neurons form anatomically segregated output circuits, each establishing topographically distinct subcircuits with their respective downstream targets.
Furthermore, the team used rabies virus-based tracing to label the inputs to MM neurons. In CBcre::PVFlp mice, helper viruses (AAV-DIO-GFP-TVA and AAV-DIO-G) were injected into the MM, and after two weeks, EnvA-RVΔG-RFP was injected into the AT region. The study found that compared to MMPV neurons, MMCB neurons received relatively fewer inputs from the hippocampus and more from the hypothalamus. Both neuron types received their strongest input from the subiculum (SUB), but with distinct topographic preferences: MMCB favored the ventral subiculum (vSUB), while MMPV favored the dorsal subiculum (dSUB), consistent with previously observed dorsoventral patterns of SUB-to-MM projections.
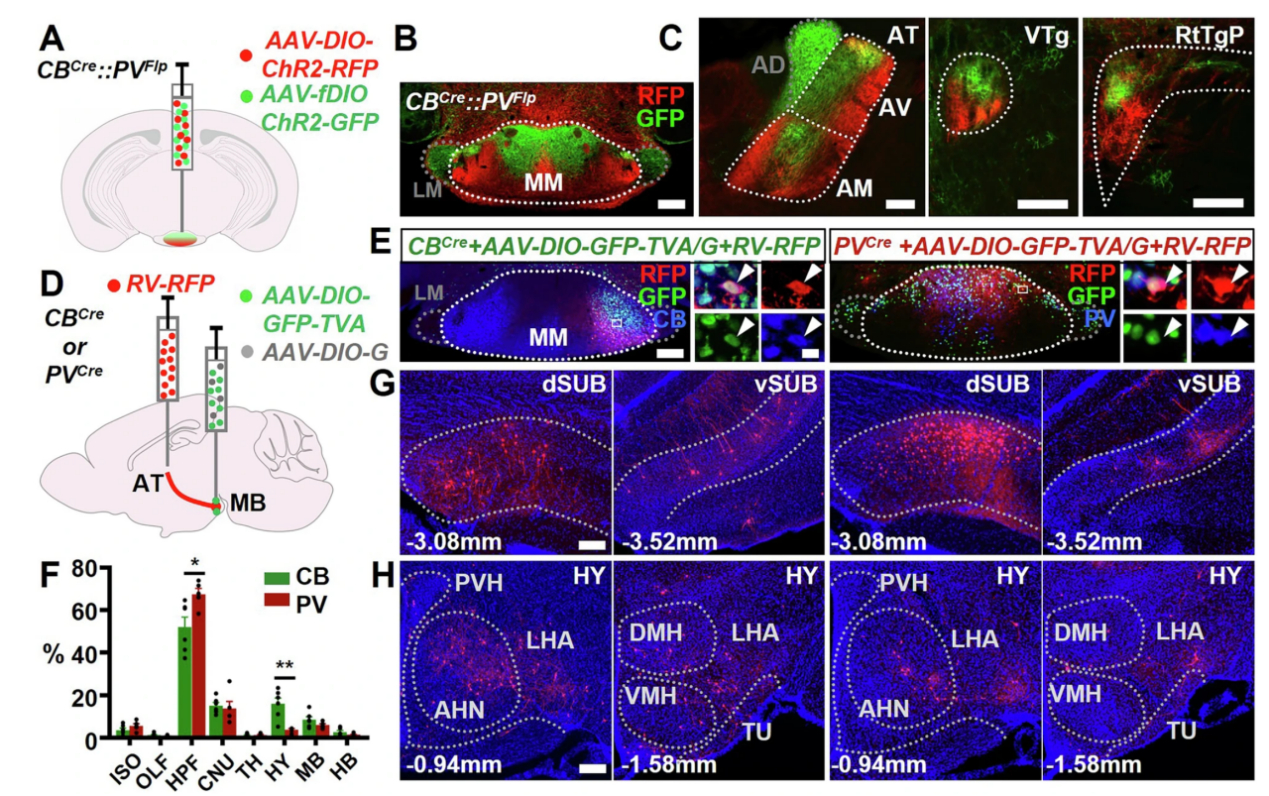
Figure 4. MMCB and MMPV Project to Complementary Brain Regions and Receive Differential Inputs
To further determine whether MMCB and MMPV neurons elicit different behavioral effects, the researchers used optogenetic techniques by injecting AAV-DIO-ChR2-RFP into the MM region of CBcre::PVcre mice. Combining this with real-time place preference and open field tests, they found that only activation of MMCB induced avoidance behavior and enhanced locomotion, while MMPV activation had no such effect.
Additionally, by injecting a calcium imaging virus (AAV-DIO-GCaMP7) into the MM region, the researchers found that calcium activity in MMCB neurons significantly increased around movement onset, with movement-related intensity 65% higher than that of MMPV neurons, which showed no significant change. These results align with the optogenetic findings, further confirming that MMCB—but not MMPV—plays a key role in motor regulation, and highlighting the functional divergence of molecularly defined neuronal subtypes.
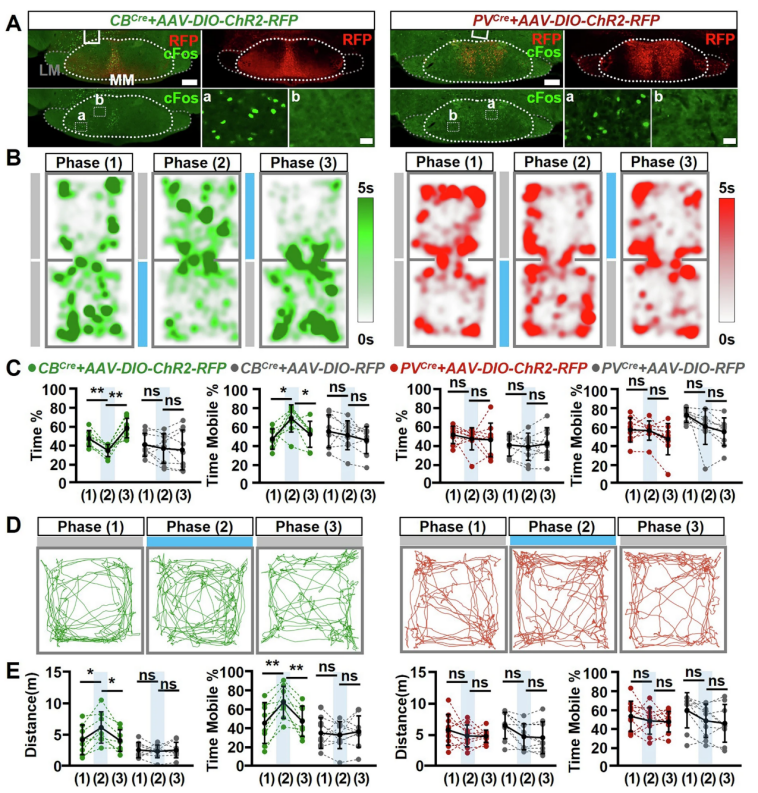
Figure 5. Optogenetic activation of MMCB—but not MMPV—induces place aversion and promotes locomotion.
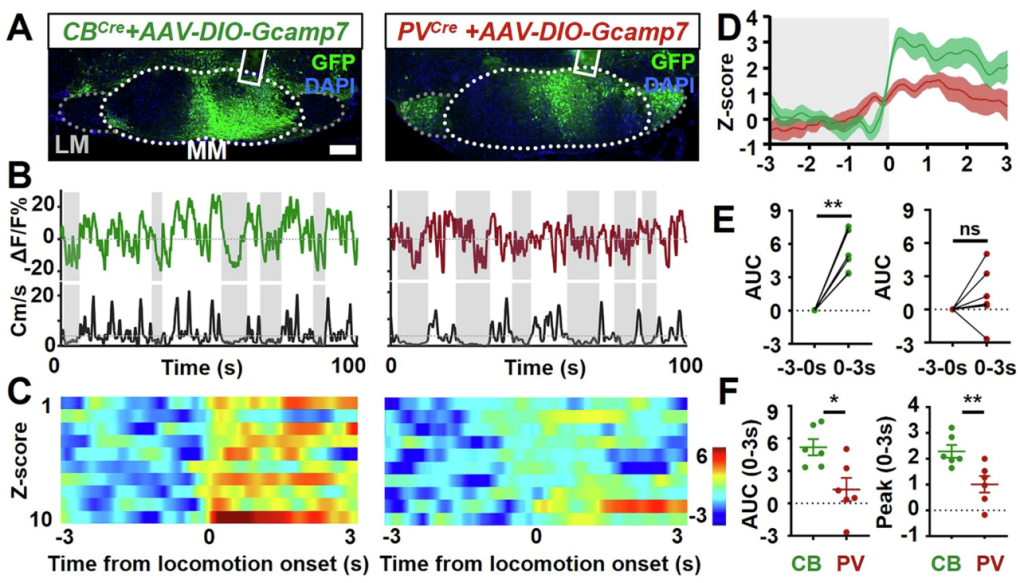
Figure 6. MMCB and MMPV mediate distinct movement-related activity.
To directly assess whether MMCB and MMPV neurons are interconnected, the researchers performed whole-cell patch-clamp recordings to measure postsynaptic responses following optogenetic activation of either MMCB or MMPV neurons. Interestingly, while direct activation could be readily detected in ChR2-expressing neurons, no optogenetically evoked postsynaptic currents (oPSCs) were observed in ChR2-negative neurons. To rule out potential NMDAR inactivation by Mg²⁺, Mg²⁺ was removed from the ACSF, yet still no oPSCs were detected in ChR2-negative neurons.
These findings further confirm that there are no synaptic connections or cross-activation between MMCB and MMPV neurons, indicating that they form independent, parallel neuronal subcircuits. Among the recorded ChR2-negative cells, some were located in the same genetically defined region as neighboring ChR2-positive cells, yet still showed no evidence of direct activation—suggesting that the lack of local connectivity among neurons within the MM is a general feature.
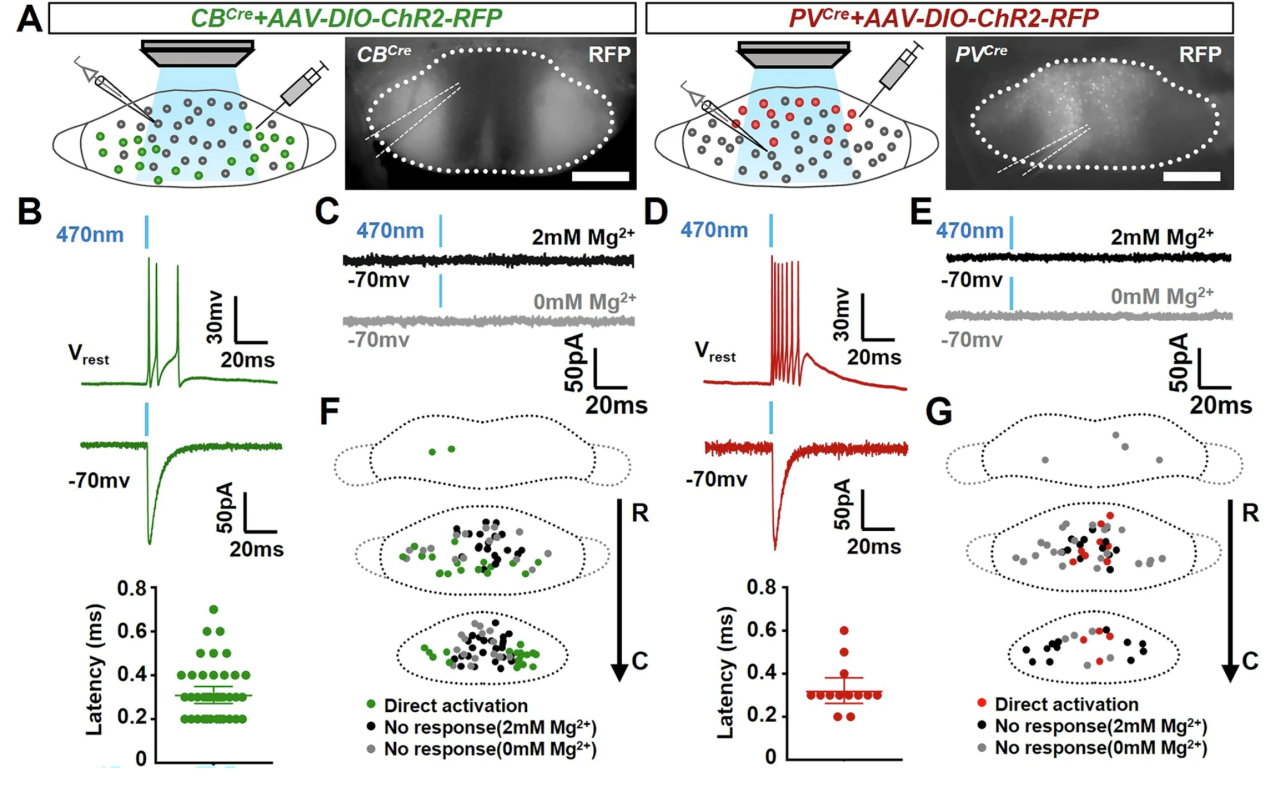
Figure 7. No Cross-Activation Observed Between MMCB and MMPV Neurons
This study is the first to systematically identify two distinct neuronal subtypes in the medial mammillary body, marked by CB and PV, and to reveal their independent, parallel circuit mechanisms and roles in behavioral regulation. The lack of local connectivity in the MM suggests that its functions are primarily mediated through long-range circuits. This research provides a cell type–specific framework for understanding the functional organization of the MM, the mechanisms underlying neuropsychiatric disorders, and potential targets for therapeutic intervention.
The viral tools used in this study were provided by Brain Case Biotech.
if you encounter any challenges during your neural circuit research, feel free to reach out to us at bd@ebraincase.com — we’d be happy to discuss and explore potential solutions with you.
