- E-mail:BD@ebraincase.com
- Tel:+8618971215294
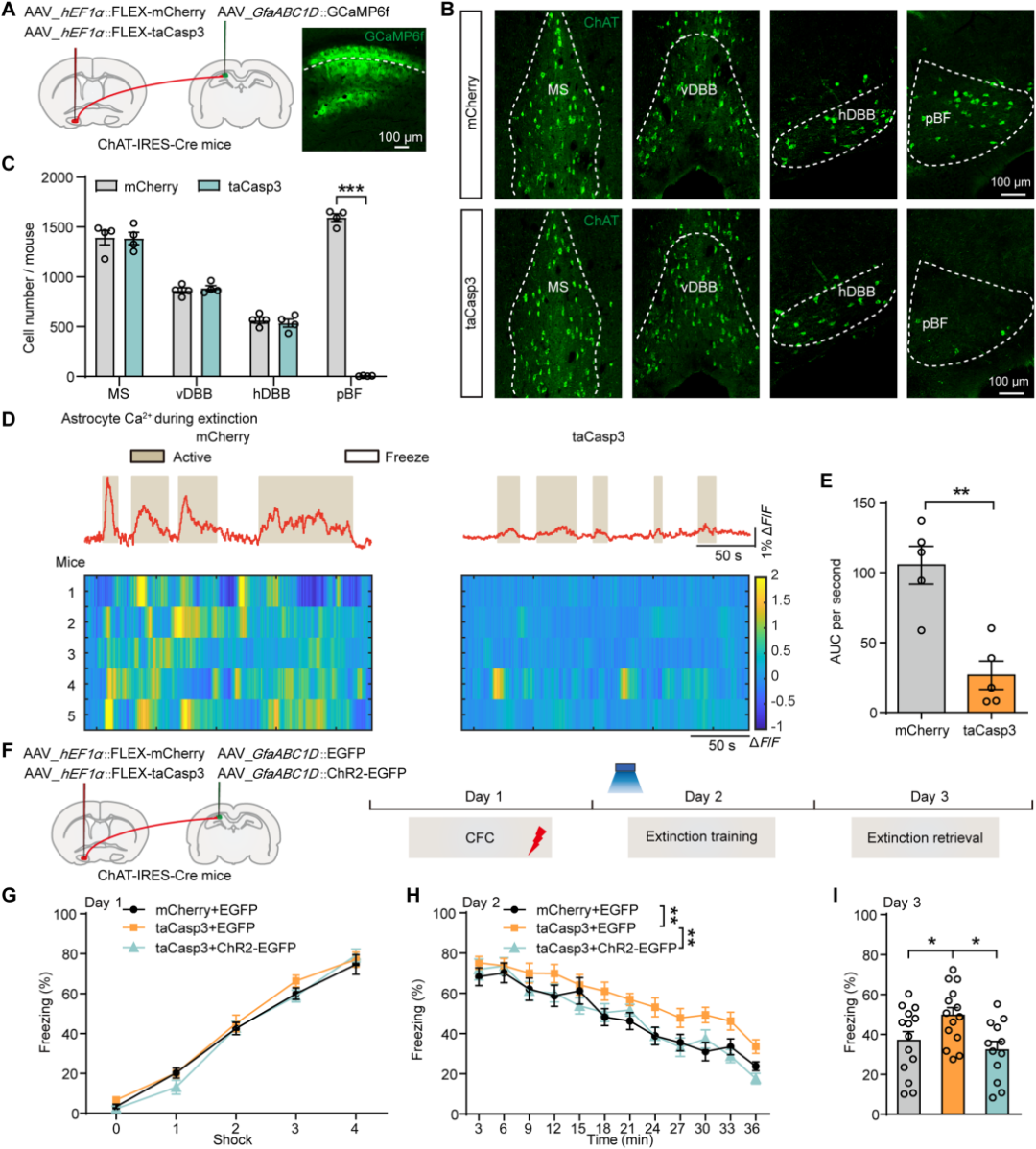
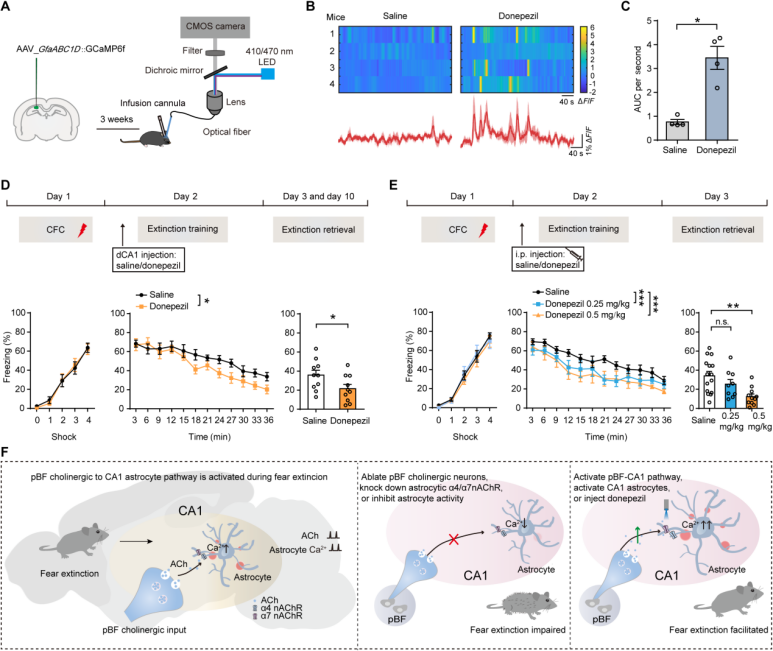
| Product Category | Product Name |
|---|---|
| Neurotransmitter Sensors | rAAV9-hSyn-g5-HT3.0 |
| rAAV5-GfaABC1D-cATP1.0 | |
| rAAV5-GfaABC1D-gACh4h | |
| rAAV9-hSyn-rAChmut | |
| rAAV5GfaABC1D-cATP1.0 | |
| Optogenetics | rAAV5-GfaABC1D-ChR2-EGFP |
| rAAV9-hEF1α-DIO-ChR2-EYFP | |
| rAAV9-hSyn-DIO-ChrimsonR-tdTomato | |
| Chemogenetics | rAAV5-GfaABC1D-hM3Dq-mCherry |
| Fluorescent Proteins | rAAV5-GfaABC1D-mCherry |
| rAAV5-GfaABC1D-EGFP | |
| Calcium Indicators | rAAV5-GfaABC1D-GCaMP6f |
| rAAV5-GfaABC1D-jRGECO1a | |
| rAAV9-hEF1α-DIO-axon-GCaMP6s | |
| Others | rAAV5-GfaABC1D-mCherry-hPMCA2w/b |
| rAAV9-hEF1α-DIO-taCasp3-TEVp |
If you are interested in the details of the experiment or possible problems and causes during the experiment, please contact: BD@ebraincase.com