Chronic Stress Impairs Intestinal Stem Cell (ISC) Function via Vagal Efferent Activation
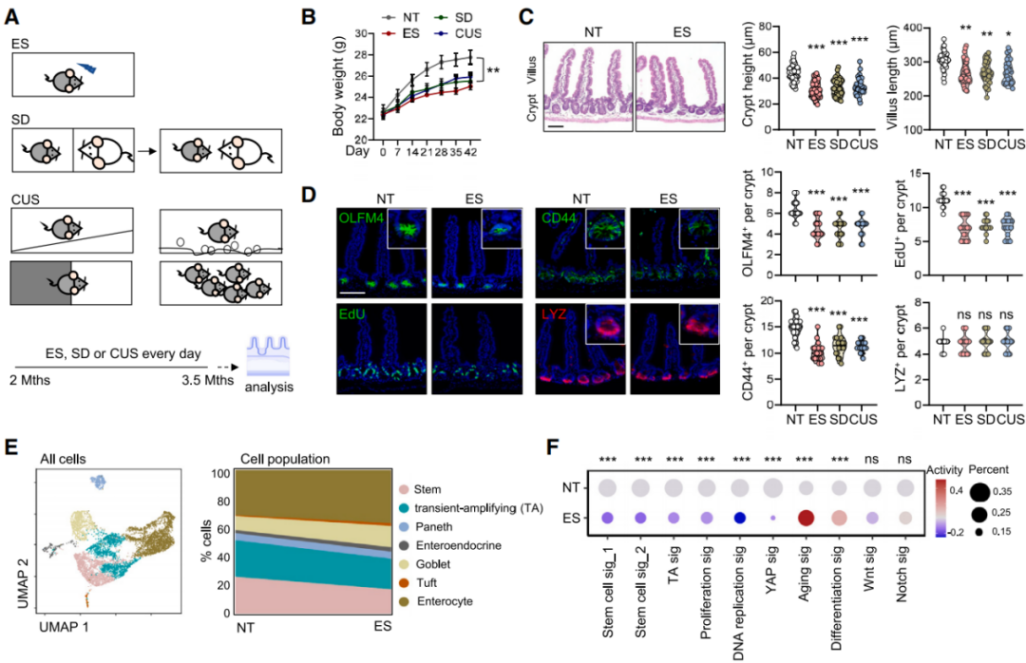
Both the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system (ANS) tightly regulate the stress response (Figure 3A). Psychological or physical stress triggers the adrenal glands to release stress hormones and catecholamines into circulation. Consistent with previous findings, the researchers observed a sustained increase in corticosterone levels in the serum of stressed mice (Figure 3B), while levels of epinephrine, norepinephrine (NA), and serotonin remained unchanged. Corticosterone binds the cytoplasmic glucocorticoid receptor (GR, also known as NR3C1), a ligand-dependent transcription factor. To determine the role of GR in ISCs, the authors generated Lgr5^EGFP-IRES-CreERT2; Nr3c1^flox/flox (Nr3c1^Lgr5-/-) mice, in which Nr3c1 is specifically deleted in Lgr5-GFP⁺ ISCs and their progeny. However, neither GR deletion nor bilateral adrenalectomy (removal of corticosterone source) mitigated the reduction in ISC number and proliferation induced by ES, suggesting that adrenal-derived steroids and catecholamines are not the primary mediators of stress-induced ISC impairment.
To explore whether the ANS mediates ISC dysfunction, the authors used an EGFP-expressing retrograde trans-synaptic pseudorabies virus (PRV) to trace brain–gut neuronal circuits (Figure 3D). The lateral paragigantocellular nucleus/rostral ventrolateral medulla (LPGi/RVLM) regulates sympathetic output to the gut via the intermediolateral column (IML) of the spinal cord and celiac ganglia, while the dorsal motor nucleus of the vagus (DMV) serves as the parasympathetic vagal center. Retrograde tracing revealed EGFP⁺ signals in LPGi/RVLM, IML, celiac ganglia, and DMV. Additionally, stressed mice showed an increase in c-FOS⁺ neurons in LPGi/RVLM. Tyrosine hydroxylase (TH)-positive sympathetic nerve fibers were found adjacent to Lgr5⁺ crypts. However, depletion of sympathetic fibers using 6-hydroxydopamine (6-OHDA), a neurotoxin targeting sympathetic neurons, reduced NA levels but did not restore ISC function (Figure 3E), indicating a minimal role for stress-induced sympathetic activation in ISC damage.
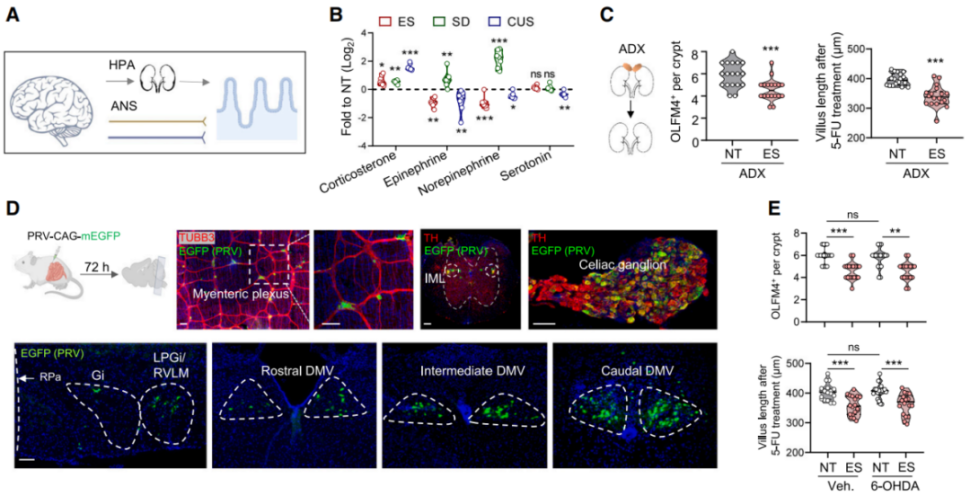 Figure 3 | HPA and Sympathetic Pathways Play Minor Roles in Stress-Induced ISC Dysfunction
Figure 3 | HPA and Sympathetic Pathways Play Minor Roles in Stress-Induced ISC Dysfunction
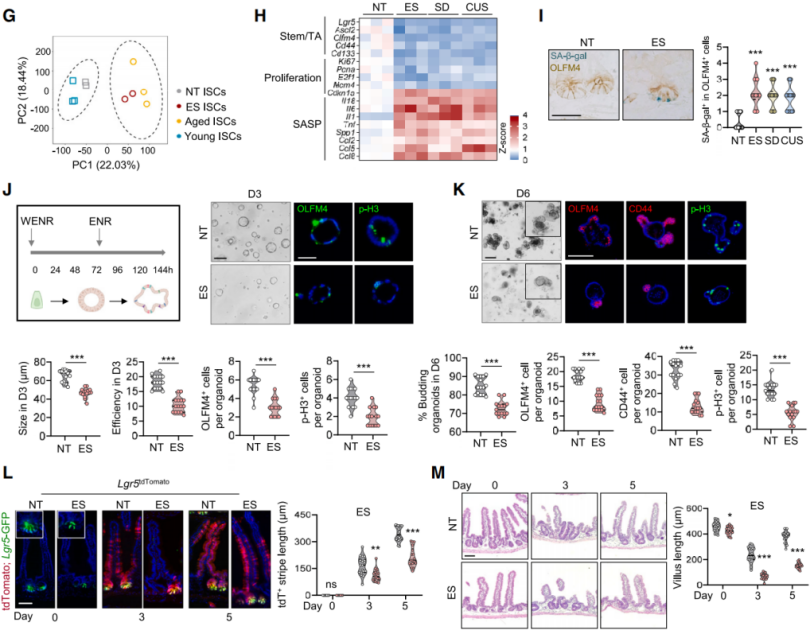
Given these findings, the researchers hypothesized that chronic stress-induced activation of the DMV and vagal efferents might impair ISC function. Since the transcription factor Phox2b is critical for vagal neuron development, they used Phox2b^Cre; Rosa26^LSL-tdTomato mice to label DMV neurons with red fluorescence. Immunostaining revealed increased c-FOS⁺tdTomato⁺ neurons in the intermediate and caudal DMV regions of stressed mice (Figure 4F), with induction strengthening over prolonged stress exposure. Whole-cell patch-clamp recordings from acute brain slices showed that ES treatment elevated excitability of Phox2b⁺ neurons, particularly increasing spontaneous firing rate and proportion of active neurons in the caudal DMV (Figures 4G–4H), suggesting that chronic stress selectively enhances vagal neuronal excitability.
To assess whether cutting vagal projections could rescue ISC function, the authors performed subdiaphragmatic vagotomy. They injected rAAV-CAG-DIO-EGFP into the caudal DMV of Phox2b tdTomato mice to selectively label vagal efferents to the gut (Figure 4I). GFP⁺tdTomato⁺ fibers marked vagal projections, whereas tdTomato-only fibers represented enteric neurons. Post-vagotomy, the number of GFP⁺tdTomato⁺ terminals near OLFM4⁺ ISCs significantly declined, while enteric neurons remained unaffected (Figure 4J). Vagotomy restored ISC number, promoted ISC regeneration after 5-FU treatment, and enhanced organoid growth (Figures 4K–4L). Lineage tracing in Lgr5 tdTomato mice further showed that vagotomy restored ISC differentiation in stressed mice (Figure 4M).
Conclusion: Chronic stress activates Phox2b⁺ neurons in the caudal DMV, increasing vagal output that negatively regulates intestinal stem cell function. Vagal pathway disruption rescues ISC stemness and regenerative capacity, highlighting a gut–brain neural mechanism in stress-induced epithelial aging.
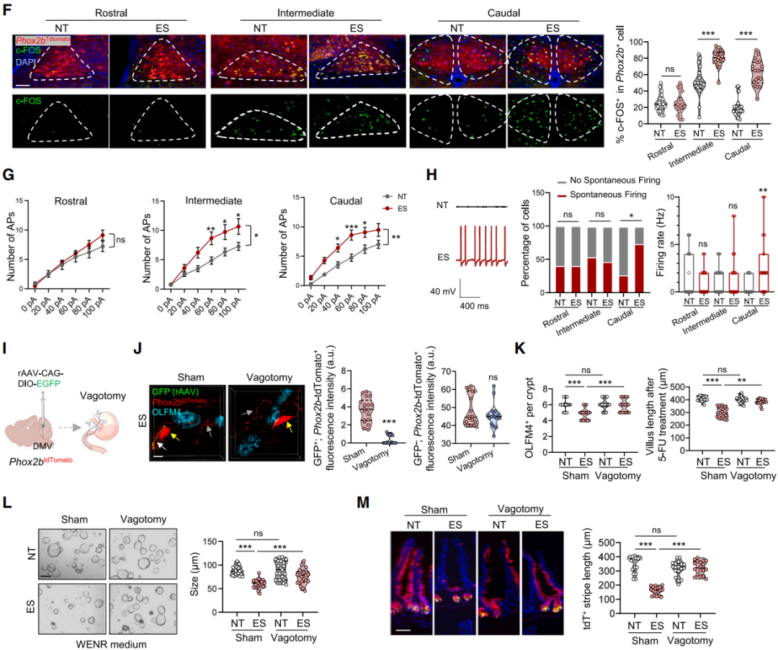 Figure 4 | Chronic Stress Impairs ISC Function via Activation of DMV Neurons and Vagal Efferents
Figure 4 | Chronic Stress Impairs ISC Function via Activation of DMV Neurons and Vagal Efferents
Vagal–Cholinergic Enteric Neurons Transmit Stress Signals via the ACh–CHRM3 Pathway
DMV neurons interact with enteric neurons to regulate various intestinal functions. To investigate whether DMV neurons directly affect ISCs or exert their effects indirectly via enteric neurons, Phox2b^DTR; tdTomato mice were generated. Phox2b is broadly expressed in enteric neurons, and PEGylated diphtheria toxin (PEGyDT)—a PEG-conjugated derivative that does not cross the blood–brain barrier—was used to selectively ablate Phox2b⁺ cells outside the central nervous system (CNS). Results showed that in Phox2b^DTR; tdTomato mice exposed to ES, peripheral Phox2b⁺ enteric neurons were ablated after PEGyDT injection, while DMV neurons remained intact (Figure 5A), which significantly restored ISC numbers and accelerated intestinal regeneration following 5-FU treatment (Figure 5B).
Similarly, co-culturing Lgr5⁺ ISCs with FACS-sorted Phox2b⁺ enteric neurons reduced organoid size, whereas ablation of Phox2b⁺ neurons by diphtheria toxin (DT) alleviated this effect (Figure 5C). These findings indicate that the vagus nerve inhibits ISC stemness via enteric neurons.
Researchers observed that the vagus nerve primarily interacts with two types of Phox2b⁺ enteric neurons—those expressing neuronal nitric oxide synthase (nNOS) or choline acetyltransferase (ChAT). Treatment of ES mice with 7-nitroindazole (7-NI), a selective nNOS inhibitor, did not improve ISC stemness. In stressed mice, intestinal acetylcholine (ACh) levels were elevated compared to controls, while nitric oxide (NO) levels showed no significant difference (Figure 5D). Notably, vagotomy and enteric neuron ablation eliminated the accumulation of ACh in the intestines of stressed mice. A 10-day administration of the ACh analog carbachol significantly reduced ISC numbers and impaired their regenerative capacity. Injection of AAV-Cre virus into the duodenal wall of Chat^flox/flox mice resulted in the deletion of ChAT⁺ enteric neurons (ChAT⁺ HuC/D⁺, Figure 5E), leading to reduced intestinal ACh levels and effective restoration of OLFM4⁺ ISC and EdU⁺ proliferative cell numbers following ES treatment (Figures 5F and 5G). These results suggest that under stress conditions, cholinergic enteric neurons release ACh, impairing ISC function.
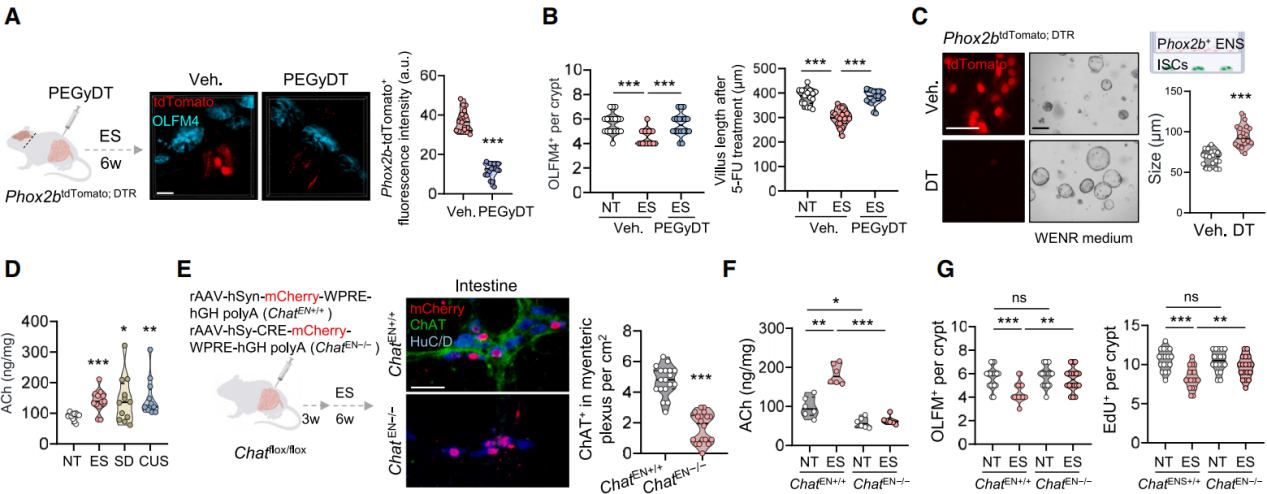
Figure 5 | Under stress, vagal activation of cholinergic enteric neurons promotes ACh release that impairs ISC function
Next, the mechanism by which vagal nerves activate cholinergic enteric neurons under stress was explored. Since ACh is the primary neurotransmitter released by vagal efferent neurons, rAAV-EF1a-DIO-GCaMP6m was injected into Phox2b-Cre mice for calcium imaging, confirming that carbachol treatment strongly stimulated enteric neurons. To confirm the role of vagal-derived ACh in enteric neuron activation, AAV-Cre virus was injected into the DMV of Chat^flox/flox mice to selectively knock out Chat in DMV neurons (Figure 6H). This led to significantly reduced ChAT expression in DMV neurons and decreased intestinal ACh levels, without affecting ChAT⁺ enteric neurons (Figures 6H–6J), and reversed stress-induced ISC defects, evidenced by restored ISC numbers and increased EdU⁺ cells (Figure 6K). These findings indicate that ACh released by the vagus nerve activates enteric neurons, triggering excessive ACh release that impairs ISC function. Although both vagal and enteric neurons release ACh, enteric neurons contribute more significantly to elevated intestinal ACh levels. The results suggest that the DMV affects ISCs indirectly via the vagus–enteric nervous system–ISC axis, in which ACh acts as a key signaling molecule.
ACh transmits signals through muscarinic (mAChRs) and nicotinic receptors (nAChRs), and Chrm3 is selectively expressed in Lgr5⁺ ISCs (Figure 6L). Treatment with mAChR agonists reduced ISC numbers and their regenerative capacity (Figure 6M). Two types of Chrm3-knockout mice were generated (Chrm3^IEC−/− and Chrm3^Lgr5−/−). Under baseline conditions, Chrm3 deletion had no effect, but in stressed mice, it restored OLFM4⁺ ISC numbers and regenerative capacity, as well as the proportion of EdU⁺ cells among Lgr5-GFP⁺ ISCs (Figure 6N). In organoid culture, Chrm3 deletion restored ISC numbers, proliferation, and differentiation capacities. Co-culture of Phox2b⁺ enteric neurons with Chrm3-deficient ISCs showed that Chrm3 deletion conferred stress resistance (Figure 6O). Transcriptomic analysis of ISCs revealed that Chrm3 deletion normalized 56% of stress-affected gene expression. Genes restored under Chrm3 deletion were enriched in stemness and proliferation-related pathways, while stress-upregulated genes were associated with calcium signaling (Figures 6P–6Q). These findings indicate that the ACh–CHRM3 signaling pathway is a stress-associated neural circuit that impairs ISC stemness.
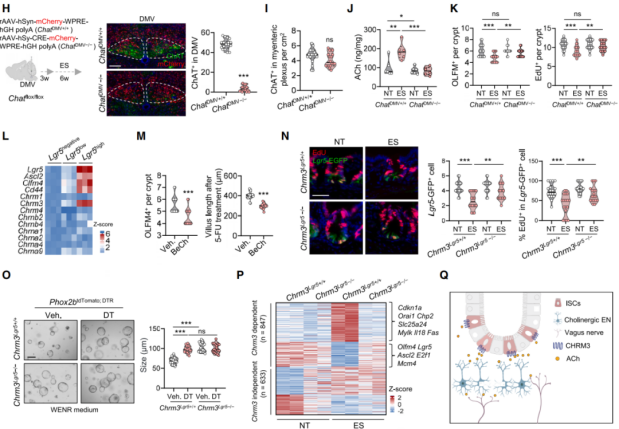
Figure 6 | The vagus–enteric cholinergic circuit impairs ISC stemness through the ACh–CHRM3 pathway under stress
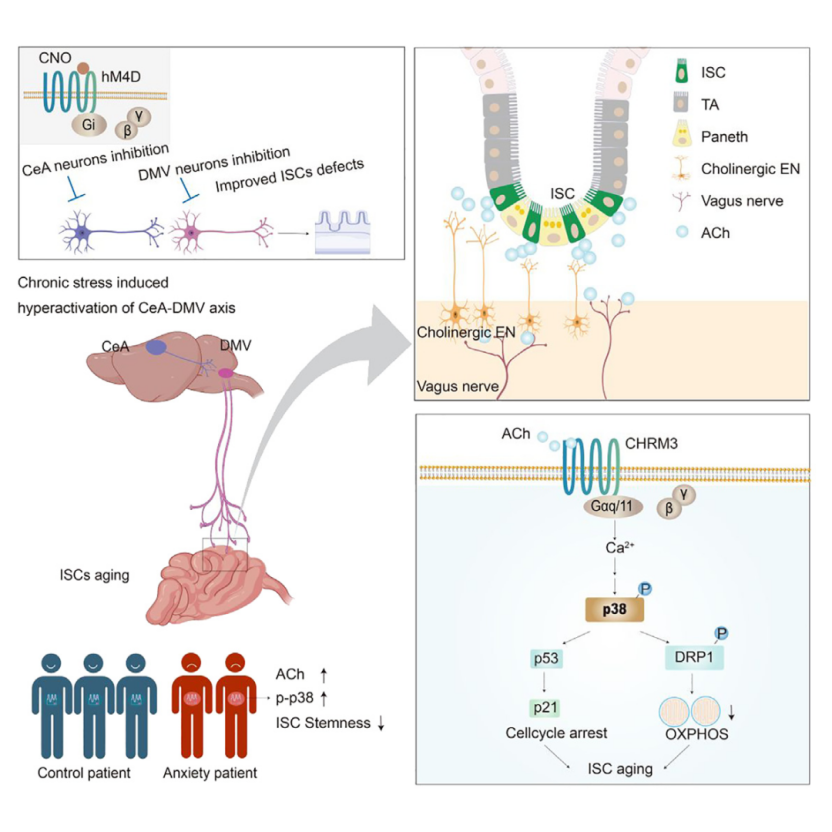
Stress-Activated ACh–CHRM3 Pathway Impairs ISC Function via Induction of the p38 Signaling Cascade
Deletion of the Chrm3 gene attenuated stress-induced transcriptomic changes in ISCs associated with aging (Figures 7A, 7B), and reduced senescence-associated β-galactosidase (SA-β-gal) staining (Figure 7C). Both the mechanistic target of rapamycin complex 1 (mTORC1) and p38 signaling pathways are known to promote ISC senescence. Immunostaining for activity markers p-S6 and p-p38 revealed a significant increase in p-p38 staining within intestinal crypts of stressed mice, while p-S6 levels remained unchanged. This p38 activation was absent in Chrm3-deficient mice (Figure 7D). Lysates from intestinal crypts of stressed mice also exhibited elevated levels of p53 and p21. Treatment with the selective p38 MAPK inhibitor SB203580 ameliorated the detrimental effects of stress on ISCs. Deletion of the Mapk14 gene, which encodes p38α, enhanced ISC numbers and regenerative capacity following 5-FU treatment in non-stressed (NT) mice (Figures 7E–F), and restored ISC stemness in stressed animals (Figures 7E–G). This also reversed stress-induced nutrient absorption defects (Figure 7H) and mitigated body weight loss (Figure 7I). Similarly, Mapk14 deletion specifically in Lgr5⁺ ISCs under stress restored ISC numbers and EdU⁺ cell proportions (Figure 8J), upregulated genes associated with stemness and proliferation, and downregulated genes linked to senescence. These findings indicate that inhibiting p38 signaling can rejuvenate intestinal stem cells in mice subjected to stress.
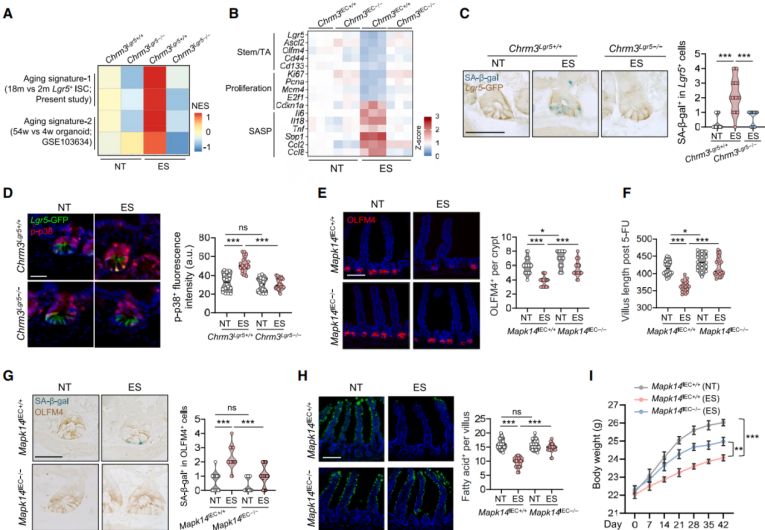 Figure 7 | Inhibition of p38 signaling rejuvenates intestinal stem cells under stress
Figure 7 | Inhibition of p38 signaling rejuvenates intestinal stem cells under stress
ACh triggers Ca²⁺ release via the Gαq/PLC/IP3 signaling pathway. In vivo two-photon calcium imaging showed that Lgr5⁺ ISCs in stressed mice exhibited increased Ca²⁺ fluctuations (Figure 8K), while BeCh-induced Ca²⁺ influx was abolished in Chrm3-deficient ISCs. Treatment with ionomycin (Iono), a calcium ionophore, reduced ISC numbers and regenerative potential (Figure 8L), and increased p-p38 levels. In contrast, inactivation of Mapk14 in ISCs prevented these Iono-induced impairments in ISC number and regeneration (Figure 8M). Tracing p38 activation back to calcium signaling, researchers found that stressed mouse ISCs had elevated levels of phosphorylated CaMKII and ASK1 (p-CaMKII, p-ASK1), indicating that Ca²⁺-dependent CaMKII–ASK1 signaling underlies stress-induced p38 activation. Similar upregulation was observed in ISCs treated with ionomycin. Furthermore, Mapk14 deficiency reduced ionomycin-induced expression of p53 and p21 (Figure 8N), suggesting that p38 acts downstream of ACh–CHRM3–Ca²⁺ signaling to drive aging-like phenotypes in ISCs.
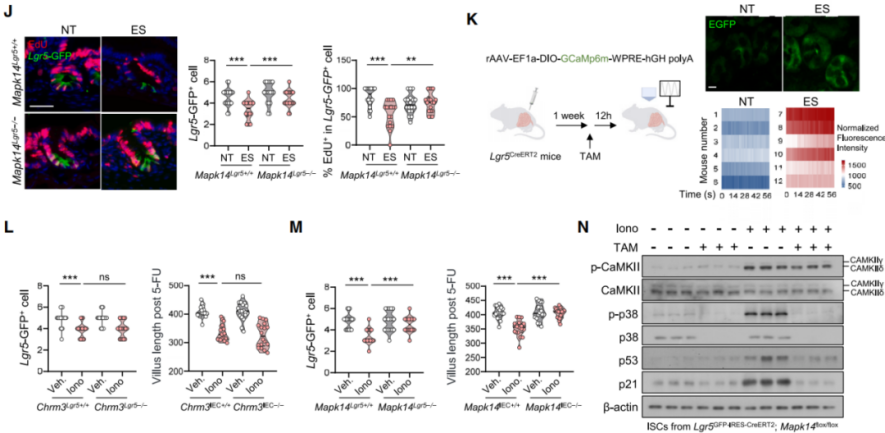 Figure 8 | p38 functions downstream of ACh-mediated calcium signaling to promote ISC aging phenotypes
Figure 8 | p38 functions downstream of ACh-mediated calcium signaling to promote ISC aging phenotypes
Chronic Stress-Induced p38 Activation Impairs Oxidative Phosphorylation
Given the role of p38 signaling in regulating the cell cycle and oxidative phosphorylation (OXPHOS), and the importance of OXPHOS in ISC function, it was hypothesized that p38 activation under stress impairs ISCs by reducing OXPHOS. Seahorse metabolic analysis revealed that ISCs from stressed mice exhibited significantly reduced oxygen consumption rates (OCR) following treatment with glucose or fatty acids (Figure 9A), with a slight increase in glycolysis (Figure 9B).
Both basal OCR and ATP production were decreased, suggesting mitochondrial dysfunction. Transmission electron microscopy and TOM20 staining further confirmed that mitochondria in stressed mouse ISCs appeared smaller and more fragmented (Figure 9D), with increased numbers of intermediate and punctate mitochondria. p38 MAPK-mediated phosphorylation of the mitochondrial fission protein dynamin-related protein 1 (DRP1) at serine 616 (p-DRP1^S616) promotes mitochondrial fission. Stress was found to enhance phosphorylation at this site (Figures 9E–F), whereas protein levels of mitochondrial fusion regulators such as MFN2 and OPA1 remained unchanged (Figure 9E). Administration of the mitochondrial fission inhibitor Mdivi-1, which blocks DRP1-dependent fission (Figure 9G), prevented mitochondrial fragmentation, increased ISC oxygen consumption in stressed mice, and partially restored stemness (Figures 9H–I). Deletion of Mapk14 in either ISCs or intestinal epithelial cells attenuated stress-induced DRP1^S616 phosphorylation (Figure 9J), promoted mitochondrial elongation, and elevated OCR (Figures 9K–L). Similar results were observed in Chrm3-deficient mice, confirming that CHRM3–p38 signaling drives DRP1^S616 phosphorylation, leading to mitochondrial fragmentation and aging-like ISC deterioration.
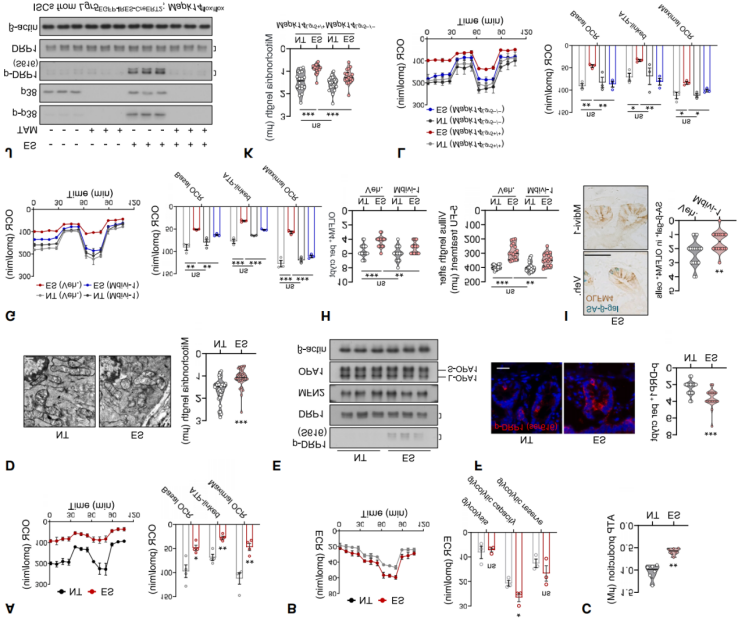
Figure 9. Chronic Stress-Induced p38 Activation Impairs Mitochondrial Function in ISCs.
Targeting the CeA-DMV Axis Alleviates Chronic Stress-Induced ISC Dysfunction
To test whether inhibiting DMV neuronal activity can mitigate the effects of chronic stress on ISCs, Cre-dependent hM4D (Gi) AAV was bilaterally injected into the DMV of Phox2b tdTomato mice (Figure 10A). Using chemogenetics to silence DMV neurons effectively reversed stress-induced ISC dysfunction, as evidenced by an increase in OLFM4+ ISCs, improved intestinal regeneration, reduced SA-β-gal+ ISCs, better organoid growth, increased EdU+ cells, and enhanced mitochondrial oxidative phosphorylation (Figures 10B–10D). In contrast, continuous activation of DMV neurons significantly reduced the number of OLFM4+ ISCs and impaired their regenerative capacity. These results indicate that DMV neuronal activity coordinates the stress response in the gut.
To explore the neural circuits involved in DMV-induced ISC dysfunction, retrograde trans-synaptic viral tracing was used to track afferent connections of DMV neurons expressing Phox2b. The virus was injected into the DMV of Phox2b-Cre mice (Figure 10E). Analysis revealed that brain regions such as the paraventricular nucleus (PVN), central amygdala (CeA), and posterior thalamic nucleus (PSTh) were RV-labeled (Figure 10F). c-FOS staining showed significant activation of PVN and CeA in the chronic stress model (Figure 10G). Chemogenetic inhibition of CeA neurons led to a significant increase in OLFM4+ cells, enhanced regenerative potential, and a reduction in SA-β-gal staining in ISCs (Figures 10H–10I). In contrast, inhibition of neurons in the PVN did not produce these effects. These results indicate that the CeA plays a critical role in mediating the stress response that affects ISC function. Using chemogenetics to inhibit or activate the CeA-DMV neural circuit (Figures 10J and 10L), the results showed that inhibition of the CeA-DMV circuit significantly increased the number of Lgr5+ ISCs, enhanced EdU incorporation, reduced activation of stress-induced p-p38, p53, and p21 signals, and maintained OXPHOS in ISCs (Figure 10K). In contrast, activation of the CeA-DMV circuit in non-stressed mice produced stress-like effects (Figure 10M). Overall, these results emphasize that the CeA-DMV circuit is a key regulatory factor in the stress response affecting ISC function.
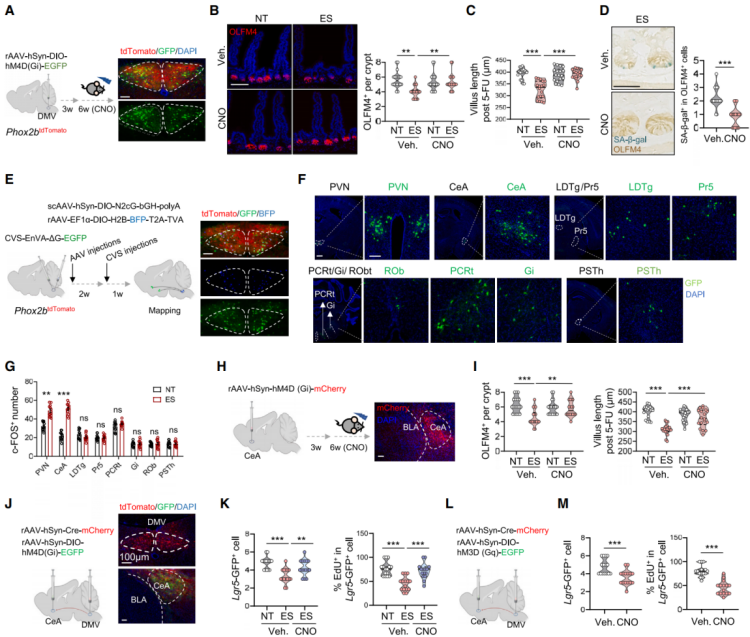 Figure 10: Targeting the CeA-DMV Axis Alleviates Stress-Induced ISC Dysfunction
Figure 10: Targeting the CeA-DMV Axis Alleviates Stress-Induced ISC Dysfunction
Stress Impairs the Stemness of Human Intestinal Stem Cells
To assess clinical relevance, we analyzed the adjacent normal tissues and serum of colorectal cancer patients, as obtaining intestinal tissues from healthy individuals was not feasible. The Generalized Anxiety Disorder 7 (GAD-7) scale was used to assess anxiety levels, the Patient Health Questionnaire 9 (PHQ-9) was used to assess depression, and the Insomnia Severity Index (ISI) was used to evaluate insomnia status, providing a comprehensive psychological health assessment. Based on these assessments, perceived stress scores were determined. Normal colon samples and serum were collected from 25 non-stressed patients and 20 stressed patients. Mass spectrometry was used to measure the levels of cortisol and ACh in the tissues and serum (Figure 11A). Cortisol and ACh levels in the intestinal tissue were positively correlated with stress scores, while only cortisol levels in the serum were significantly correlated with stress scores (Figures 11B–11C). Immunofluorescence showed that stem and proliferating cells (OLFM4 and Ki67 staining) were significantly reduced in the colon tissue of stressed patients, and p-p38 signaling was more intense (Figures 11D–11F). qPCR analysis indicated that the expression of stem cell markers (LGR5, OLFM4, ASCL2) and TA/proliferation genes (CD44, CD133, KI67) were downregulated, while genes associated with aging/SASP were upregulated in stressed patients. These findings suggest that stress may also impair the stemness of human intestinal stem cells through similar mechanisms.
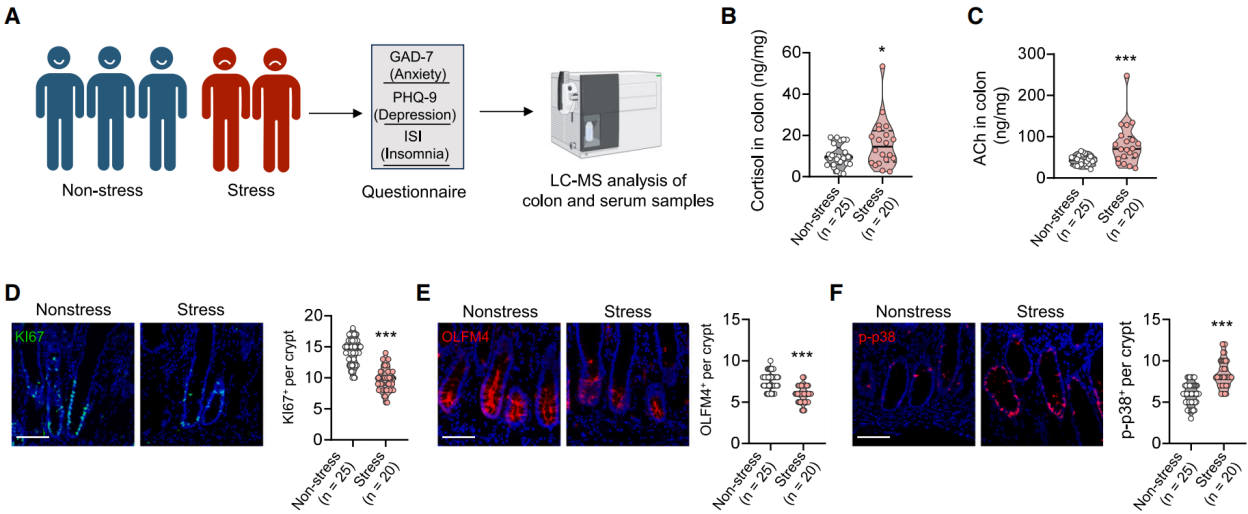 Figure 11: Psychological Stress Affects Human Intestinal ACh Levels and Proliferation Defects
Figure 11: Psychological Stress Affects Human Intestinal ACh Levels and Proliferation Defects
Summary
This study investigates the impact of chronic stress on ISCs and confirms that chronic stress impairs the stemness of ISCs, reducing their self-renewal and differentiation abilities, ultimately leading to intestinal regeneration defects. Stress primarily acts through the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system. Activation of the dorsal motor nucleus of the vagus nerve (DMV) induces vagal pathway activation, which, through the release of acetylcholine by enteric neurons, damages ISC function. The study also highlights the role of the CeA-DMV neural axis formed by the central amygdala (CeA) and DMV in regulating the impact of stress on ISC function. Furthermore, analysis of colorectal cancer patients suggests that similar mechanisms exist in humans.
The viral tools used in this study are available through Brain Case Biotech.