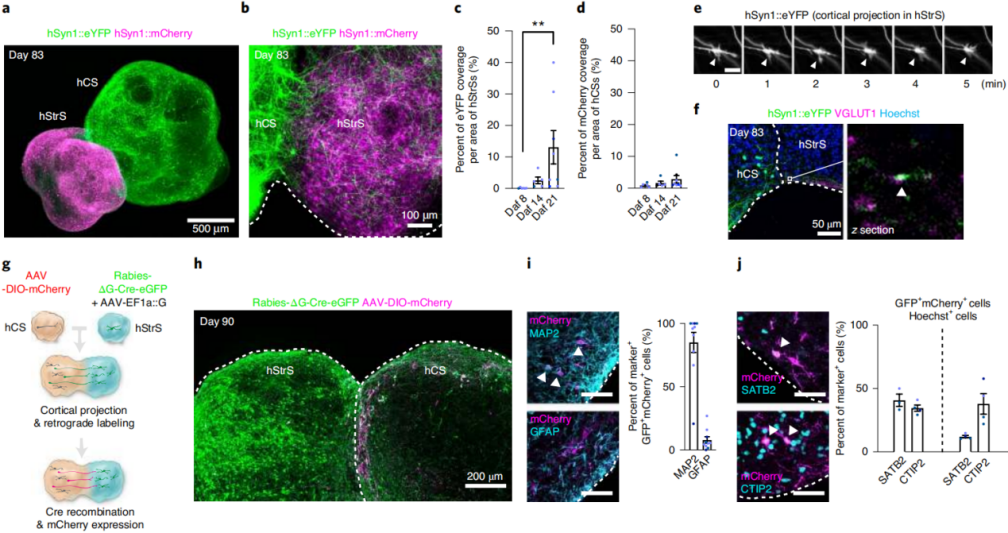
Functional Neural Circuits in Cortico-Striatal Assembloids
To determine whether hCS projection neurons formed functional synaptic connections with hStrS neurons, the researchers combined optogenetics with simultaneous calcium imaging in cortico-striatal assembloids. For light-evoked calcium responses, hCSs were transduced with hSyn-ChrimsonR–tdTomato (a red-shifted opsin), while hStrSs were co-infected with Ple94-iCre and DIO-GCaMP6s (Fig. 2a). Two days after viral infection, hCSs and hStrSs were assembled, and imaging was performed at ~90 days of differentiation (Fig. 2b). Upon 625 nm light stimulation, robust calcium responses were observed in hStrS cells (Fig. 2c,d). The median ΔF/F of GCaMP6 signals after light stimulation was significantly higher than at random time points. While responsive cells reliably tracked varying light frequencies, non-responsive or spontaneously active cells were also detected (Fig. 2d). The AMPA receptor antagonist NBQX (20 μM) and NMDA receptor antagonist APV (50 μM) blocked the light-induced calcium responses (Fig. 2d,e), with effects reversible upon washout, indicating that part of the calcium activity was mediated by glutamatergic transmission from hCSs to hStrSs. Notably, the median amplitude of light-evoked GCaMP6 signals increased at days 100–120 and 140–150 compared to day 100, suggesting that synaptic strength or frequency within cortico-striatal assembloids strengthens over time.
To further characterize connectivity, optogenetic stimulation was combined with whole-cell patch-clamp electrophysiology in acute slices (Fig. 2f). Green-light (550 nm) stimulation induced optogenetically evoked excitatory postsynaptic currents (oEPSCs), postsynaptic potentials (oEPSPs), and spiking in hStrS neurons (Fig. 2g). Out of 35 recorded neurons, 11 responded (Fig. 2h), with an average oEPSC amplitude of ~40 pA (Fig. 2i) and an onset latency of ~6 ms (Fig. 2j), confirming the establishment of functional synapses between hCS projection neurons and hStrS neurons. Corticostriatal glutamatergic inputs are critical for the maturation of medium spiny neurons. To assess whether assembloid formation influenced electrophysiological properties, whole-cell patch-clamp recordings were performed on hStrS neurons within the assembloids (Fig. 2k). Compared with age-matched standalone hStrSs, hSyn-eYFP–labeled hStrS neurons in the assembloids exhibited increased intrinsic excitability (Fig. 2l–n), while action potential amplitude and threshold remained unchanged, consistent with findings from developing mouse striatum. Collectively, these results demonstrate that human iPSC-derived cortico-striatal assembloids support functional connectivity and alter intrinsic firing properties of hStrS neurons.
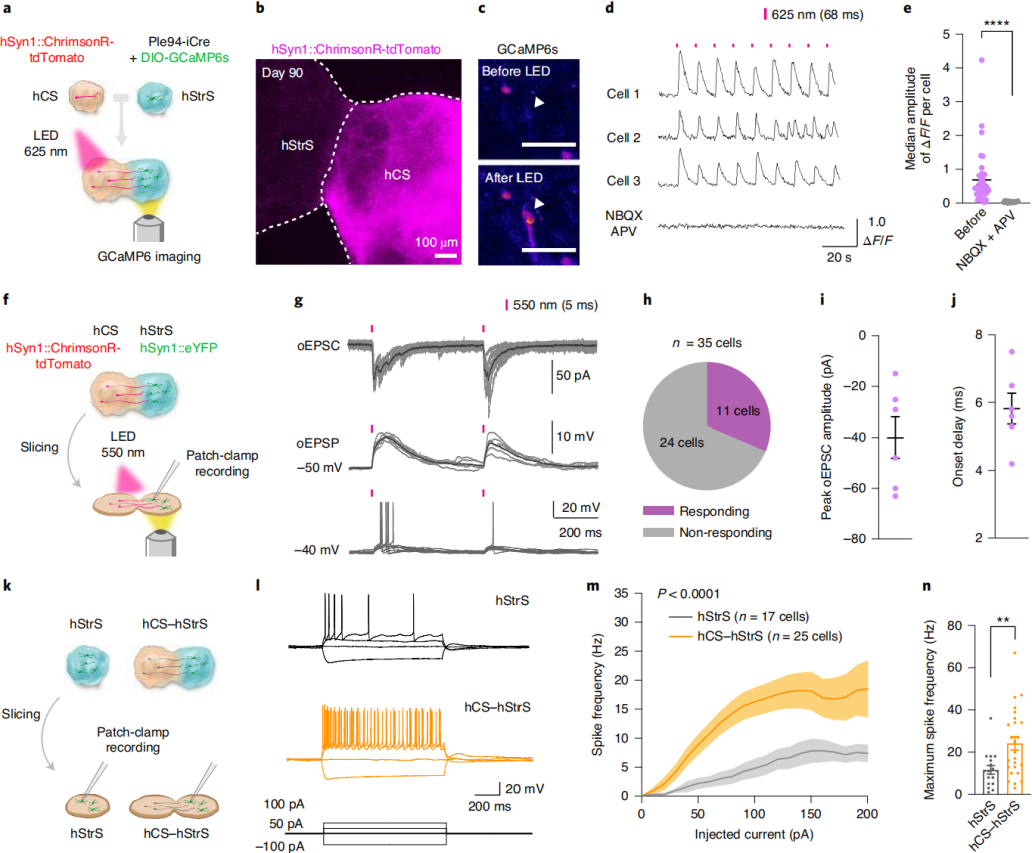 Figure 2. Functional neural circuits in cortico-striatal assembloids
Figure 2. Functional neural circuits in cortico-striatal assembloids
Modeling Disease-Related Cellular Phenotypes in Cortico-Striatal Assembloids:To assess whether cortico-striatal assembloids can model cellular deficits associated with genetic neurodevelopmental disorders, researchers focused on Phelan–McDermid syndrome (22q13.3 deletion syndrome). This condition results from deletion of chromosome 22q13.3, which includes the SHANK3 gene encoding a postsynaptic scaffolding protein. SHANK3 is highly expressed in the striatum and is critical for the development and function of cortico-striatal circuits; its loss is a major pathogenic factor. While Shank3 knockout mice display cortico-striatal connectivity defects, whether heterozygous patient-derived human cells exhibit similar abnormalities has remained unclear.
iPSC lines from three 22q13.3DS patients were differentiated into hCSs and hStrSs (Fig. 3a). Patient-derived cells formed hStrSs in 3D culture (Fig. 3b), expressed forebrain and LGE markers, and lacked spinal cord markers. The size of patient-derived hCSs and hStrSs was comparable to controls (Fig. 3c), and they generated DARPP32⁺NeuN⁺ neurons (Fig. 3d). When cortico-striatal assembloids were generated from three patients and three healthy controls and subjected to calcium imaging (Fig. 3e–g), patient-derived neurons in the hStrS region exhibited increased calcium spike events (Fig. 3e–g). Notably, standalone patient hStrSs at the same stage did not show this defect (Fig. 3h–j). Calculation of mean intercellular correlation coefficients revealed reduced network synchrony in both patient-derived hStrSs and assembloids (Fig. 3k–m). In conclusion, cortico-striatal assembloids can faithfully recapitulate abnormal neural activity of human cells derived from patients with 22q13.3 deletion syndrome.
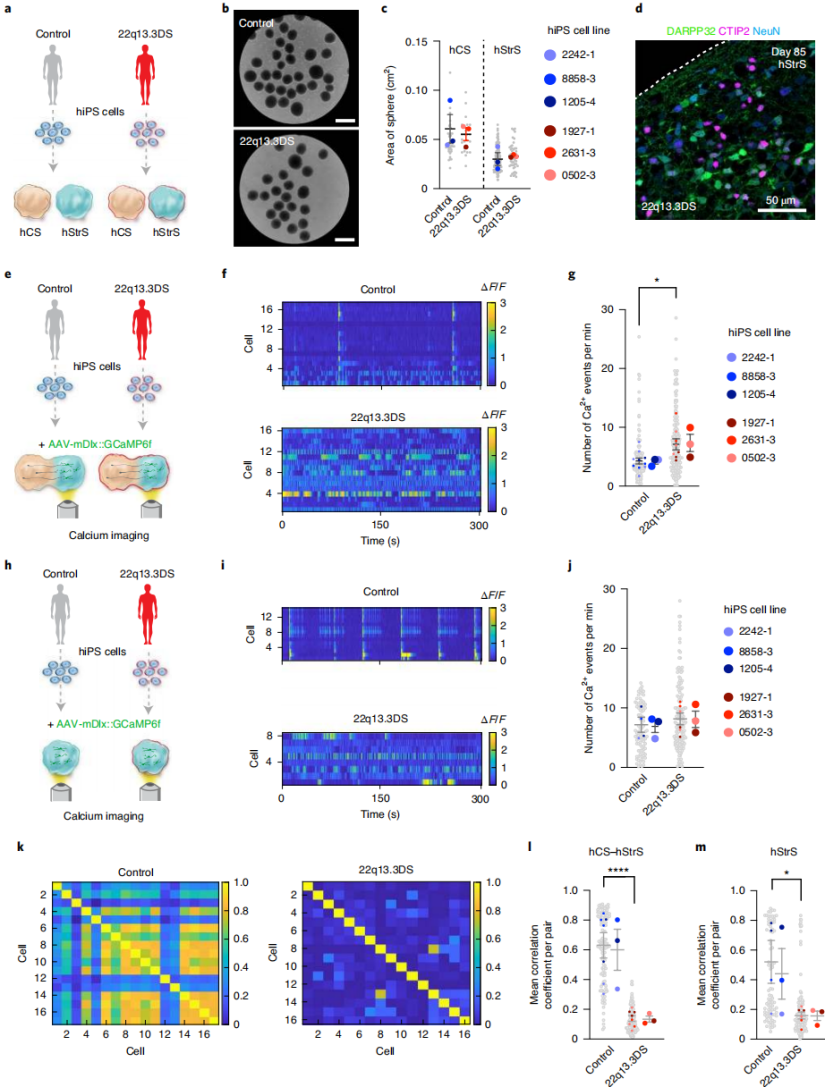 Figure 3. Cortico-striatal assembloids reveal altered neuronal activity in 22q13.3DS patient-derived cells
Figure 3. Cortico-striatal assembloids reveal altered neuronal activity in 22q13.3DS patient-derived cells
Conclusion
This study established human striatal organoids containing functional medium spiny neurons from human pluripotent stem cells by modulating signaling pathways, and assembled them with cortical organoids to generate cortico-striatal assembloids. Retrograde viral tracing identified the types of cortical neurons projecting to the striatum; optogenetics combined with calcium imaging confirmed the formation of functional glutamatergic synaptic connections between the two regions, and assembly promoted the maturation of striatal neurons. Furthermore, assembloids generated from patient-derived cells with 22q13.3 deletion syndrome exhibited disease-related calcium activity abnormalities and reduced network synchrony. These findings demonstrate that this model—integrating retrograde viral tracing, optogenetics, and calcium imaging—provides an effective platform for studying human cortico-striatal circuit development and disease mechanisms.