- E-mail:BD@ebraincase.com
- Tel:+8618971215294
Spinal cord injury (SCI) is a serious disease of the central nervous system. After spinal cord injury, a large number of motor neurons are lost at the injury site, while astrocytes proliferate reactively and form dense glial scars. Modulating the density of glial scars and replenishing motor neurons in the injured area are critical for spinal cord injury repair. Polypyrimidine tract-binding protein (PTB) is a ribonucleic acid-binding protein involved in neurogenesis. Studies have found that in mouse models of Parkinson's disease and aging, polypyrimidine tract binding protein knockdown can replenish neurons in the brain and reverse the motor phenotypes of mice. Inhibiting the expression of PTB is a powerful strategy to treat various diseases caused by neuronal loss, but the role of PTB knockdown in spinal cord injury repair has not yet been explored.
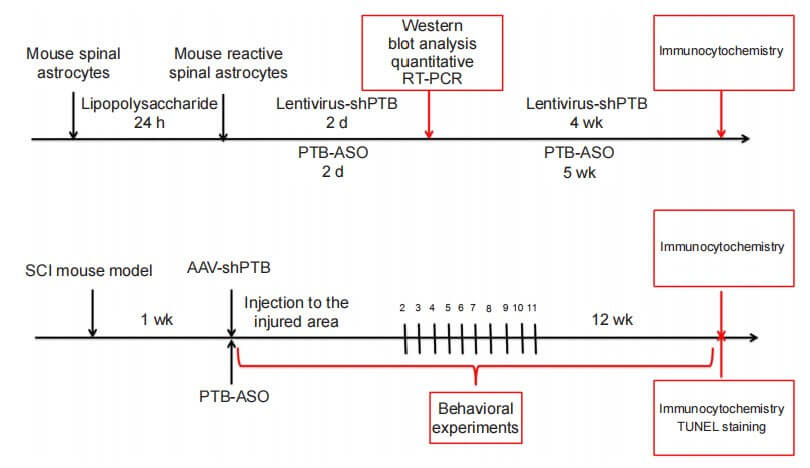
Chen Gang's team from Nantong University published a paper "Knockdown of polypyrimidine tract binding facilitates protein motor function recovery after spinal cord injury" in "Chinese Neuroregeneration Research (English version)" using short hairpin RNAs (shRNAs) and antisense oligos carried by the virus. nucleotides (ASOs) to knock down the expression of polypyrimidine tract-binding proteins to determine its role in mouse spinal cord reactive astrocytes and spinal cord injury mouse models.
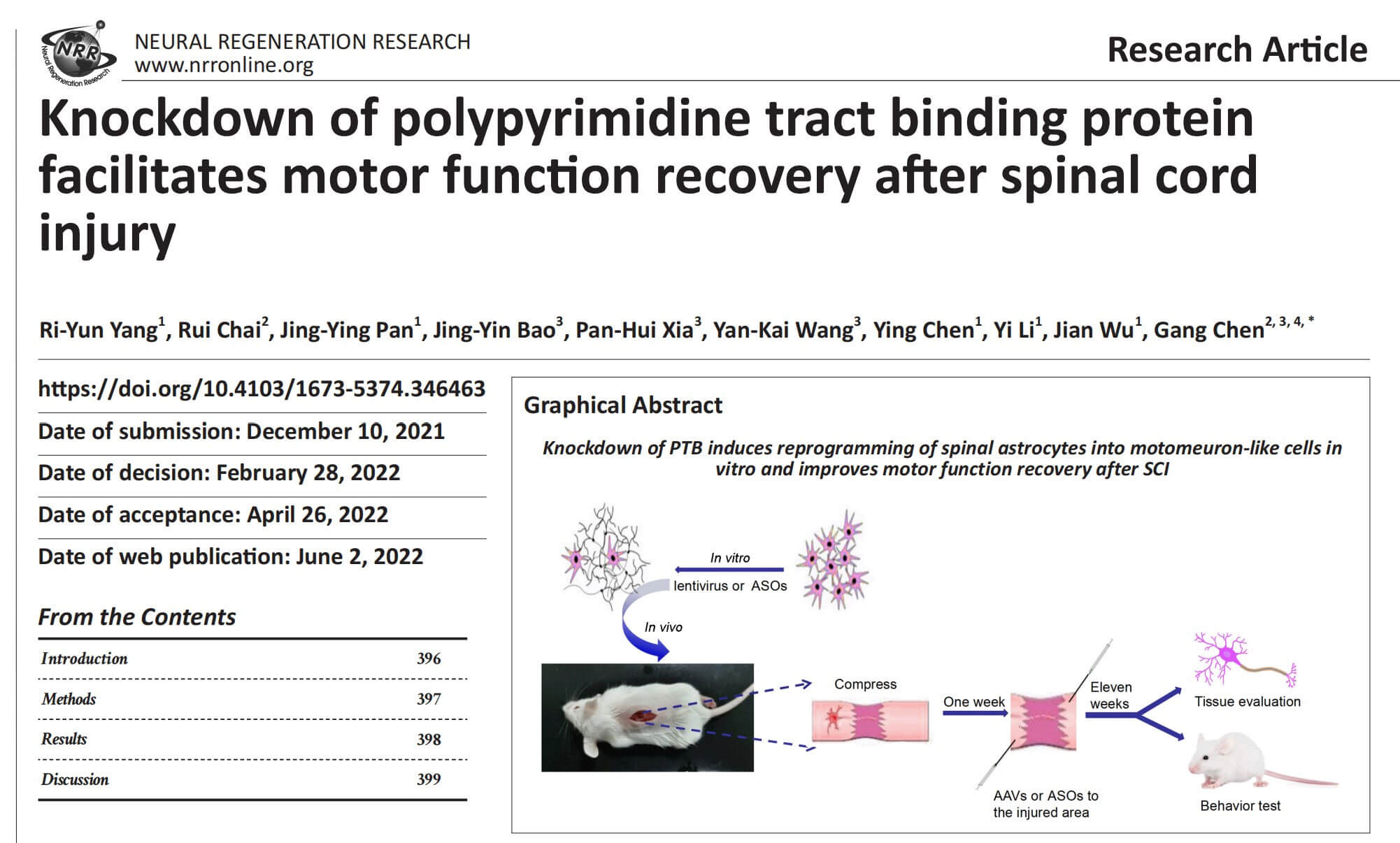
The authors explored whether PTB silencing causes reactive mouse spinal cord astrocytes to convert into motor neurons in vitro. Mouse reactive spinal cord astrocytes were transduced with a lentiviral vector containing the GFAP promoter to drive expression of shRNA against mouse Ptbp1. When astrocytes were transduced with this vector for 2 days, shPTB significantly down-regulated PTB protein expression and Ptbp1mRNA expression. At 2 weeks after reprogramming, shPTB-transduced cells showed complex neurite outgrowth, and by 4 weeks, these cells showed distinct neuronal morphology, whereas shCtrl-transduced cells showed astrocyte-like flattened and polygonal shapes. form. Three weeks after transduction, approximately 41% of GFP-positive cells transduced with shPTB showed positive staining for the neuronal marker MAP2. Four weeks after transformation, the percentage of GFP+/MAP2+ cells was approximately 58%, and approximately 12% of GFP+ cells showed expression of the motor neuron marker ChAT. In contrast, cells transduced with the shCtrl lentiviral vector only showed positive staining for GFP. These data demonstrate that reactive spinal cord astrocytes can be reprogrammed into motor neuron-like cells by PTB silencing in vitro.
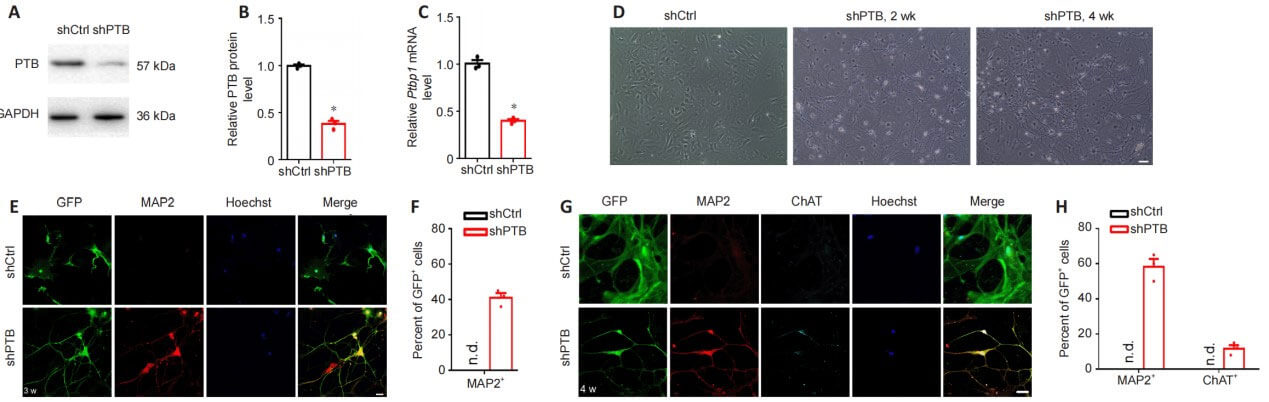
To determine whether PTB silencing could replenish neurons, including motor neurons, in a mouse model of spinal cord injury, the authors injected AAV diagonally contralateral to the injured spinal cord region. The GFAP promoter was used to drive the expression of shPTB. One week after AAV-shPTB injection, a large number of GFP+ cells co-localized with GFAP+ astrocytes, but rarely co-localized with ChAT+ motor neurons, and did not co-localize with FoxJ1+ neural stem cells. . Eleven weeks after injection, some GFP+ cells co-localized with neuronal markers NeuN or MAP2 around the injured area, and approximately 19% of GFP+ cells expressed the motor neuron-specific marker ChAT. At the same time point, after injection of AAV-shCtrl, no GFP+ neuron-like cells and GFP+ motor neuron-like cells were detected around the injured area. Compared with the AAV-shCtrl group, the number of NeuN+, MAP2+ and ChAT+ cells was more abundant in the AAV-shPTB group. Taken together, these results suggest that shPTB can replenish motor neuron-like cells surrounding the injured area after spinal cord injury.
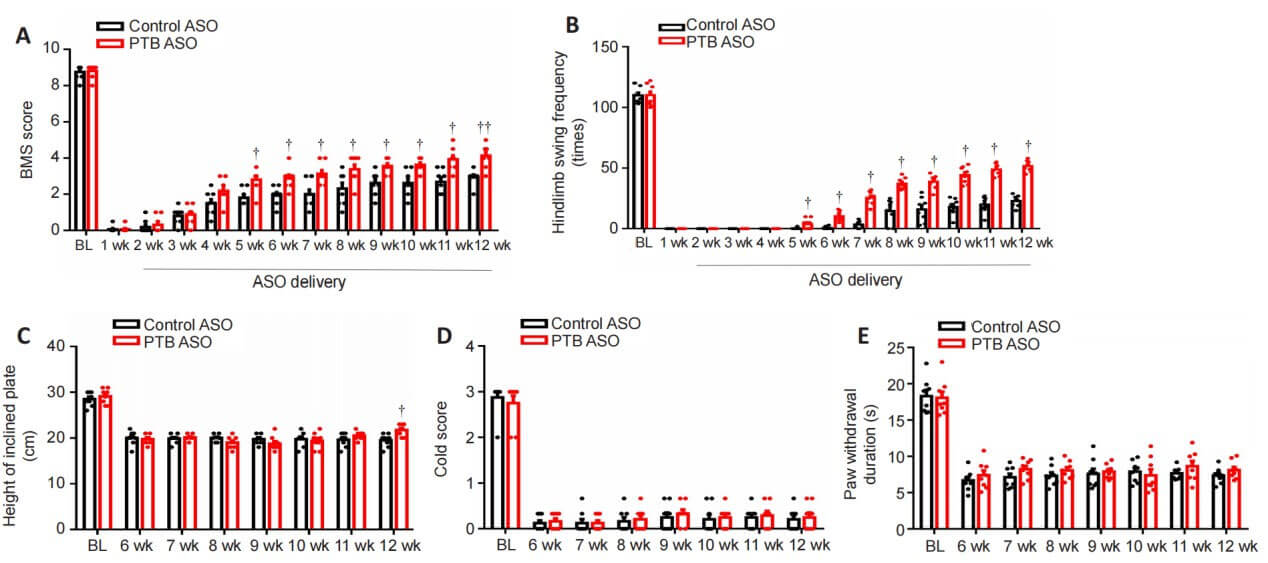
To determine whether PTB knockdown can promote functional recovery in spinal cord injury mice, the authors used BMS scores and swimming tests to evaluate motor function recovery. Compressive spinal cord injury was induced in mice, and AAV-shPTB or AAV-shCtrl was injected in situ 1 week after injury. At 6-12 weeks after injury, BMS score, swimming test and tilt plate test results showed that recovery of muscle strength was improved after PTB gene knockout. The results of cold stimulus-induced pain test or hot plate test showed that the performance of mice injected with AAV-shPTB did not improve compared with that of mice injected with AAV-shCtrl. These results suggest that AAV-shPTB treatment can improve motor function after spinal cord injury.
As shown in the figure, compared with the control group, the expression of PTB protein and Ptbp1 mRNA in astrocytes in the PTB-ASO transfection group was significantly reduced 2 days after transfection. Five weeks after transfection, the morphology of the reprogrammed cells and the expression of the mature neuron marker MAP2 and the motor neuron marker ChAT were assessed by immunocytochemistry. The results showed that PTB-ASO could significantly induce the transformation of reactive spinal cord astrocytes into motor neuron-like cells in vitro.
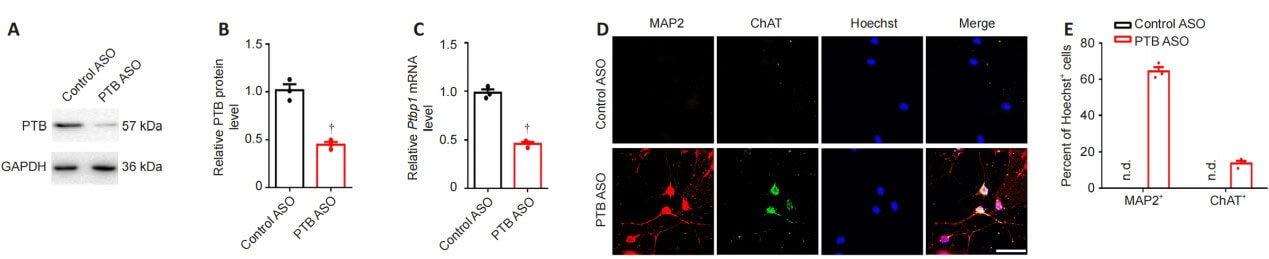
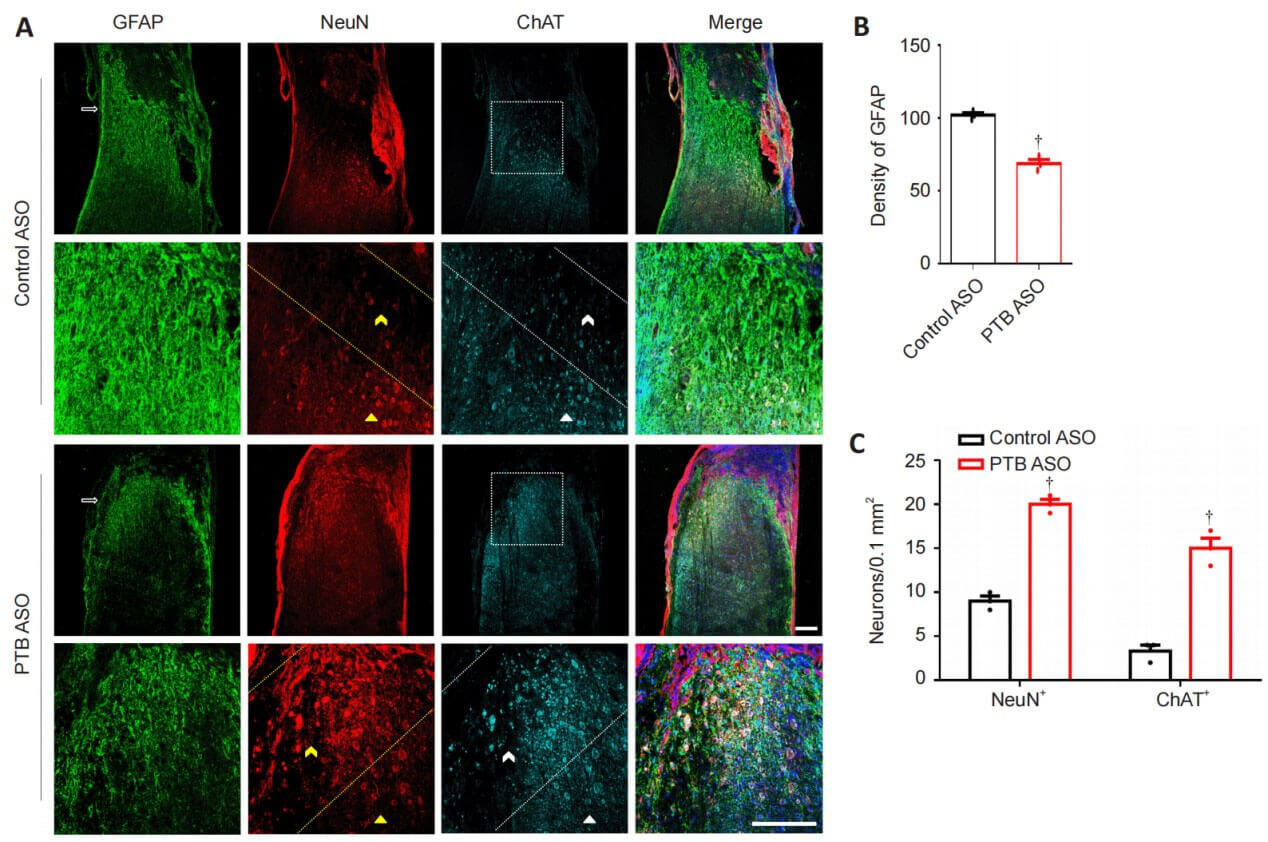
The authors verified the effect of PTB ASOs on the density of glial scar in the spinal cord by staining GFAP in the injured spinal cord. At 12 weeks after spinal cord injury, control mice injected with ASO showed typical glial scar morphology and interlaced astrocytes. Compared with the control group, the astrocytes derived from glial scars in mice in the PTB ASO injection group were loosely distributed, had fewer protrusions, and the density of glial scars was reduced. Twelve weeks after spinal cord injury, compared with the control group, mice in the PTB-ASO injection group had more NeuN+ and ChAT+ cells in the proximal area of the lesion area. TUNEL staining and quantitative RT-PCR results showed that PTB-ASO moderately reduced the glial scar density in the spinal cord injury area without destroying its overall structure, replenished peripheral motor neuron-like cells, and reduced cell apoptosis in the spinal cord injury area. Death.
The function of PTB-ASO was further evaluated through behavioral tests on spinal cord injury mice. The results of BMS score, swimming test and tilt plate test showed that compared with the control ASO-treated group, the motor function of mice in the PTB-ASO-treated group was improved 5-12 weeks after spinal cord injury. Twelve weeks after injury, mice in the PTB-ASO-treated group tended to use their lower limbs and showed greater muscle strength after spinal cord injury compared with the control group. There were no differences in cold- and heat-induced sensory responses between PTB-ASO-treated and control ASO-treated mice 6-12 weeks after injury. These results indicate that PTB-ASOs promote the recovery of motor function in spinal cord injury model mice.
In a mouse model of compressive spinal cord injury, knockdown of polypyrimidine tract-binding protein by adeno-associated virus short hairpin RNA can increase the number of motor neuron-like cells near the injured area and promote the recovery of motor function in mice; while antisense Oligonucleotide knockdown of the expression of polypyrimidine tract-binding protein not only improves motor function recovery in spinal cord injury mice and replenishes motor neuron-like cells around the injured area, but also moderately reduces collagen without damaging its overall structure. Density of scar tissue. This result indicates that polypyrimidine tract binding protein knockdown may be an effective strategy for the treatment of spinal cord injury.
The following interpretation only represents Xiaobu's personal understanding and opinion of the article. All the pictures used in this interpretation are from the original text. If you need more information, please refer to the original literature