- E-mail:BD@ebraincase.com
- Tel:+8618971215294
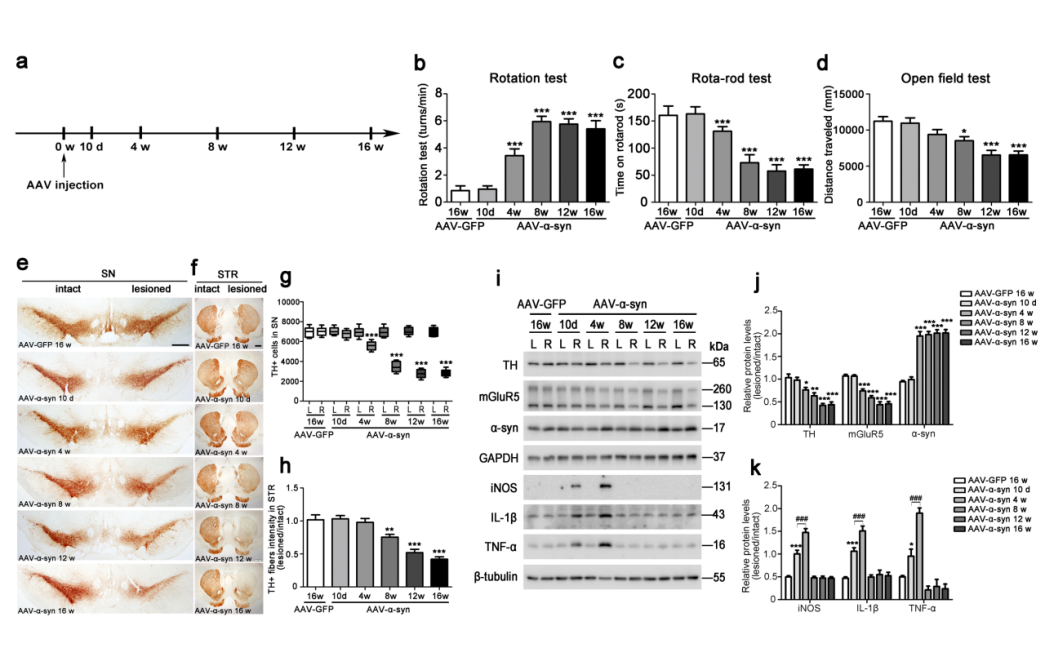
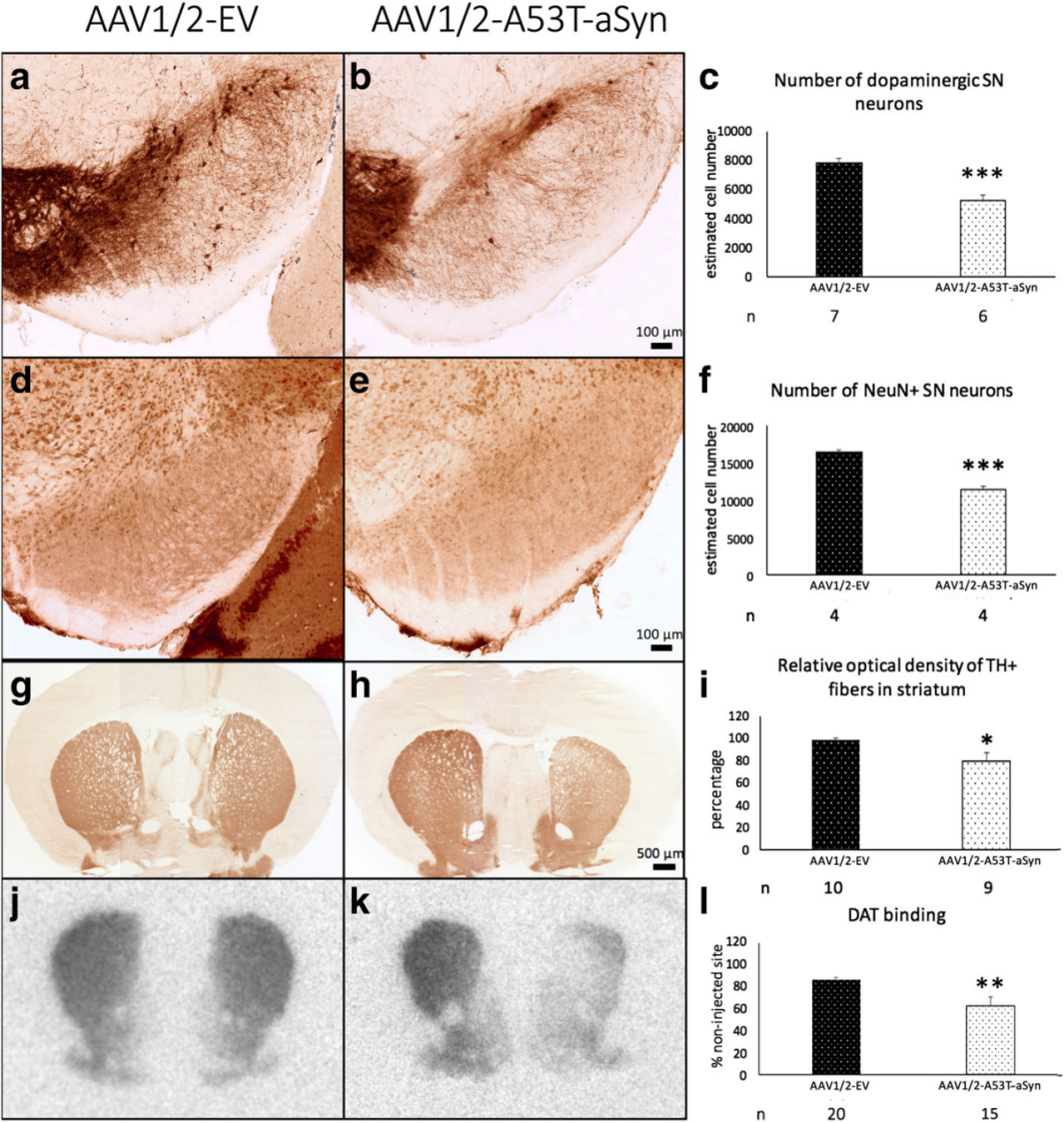
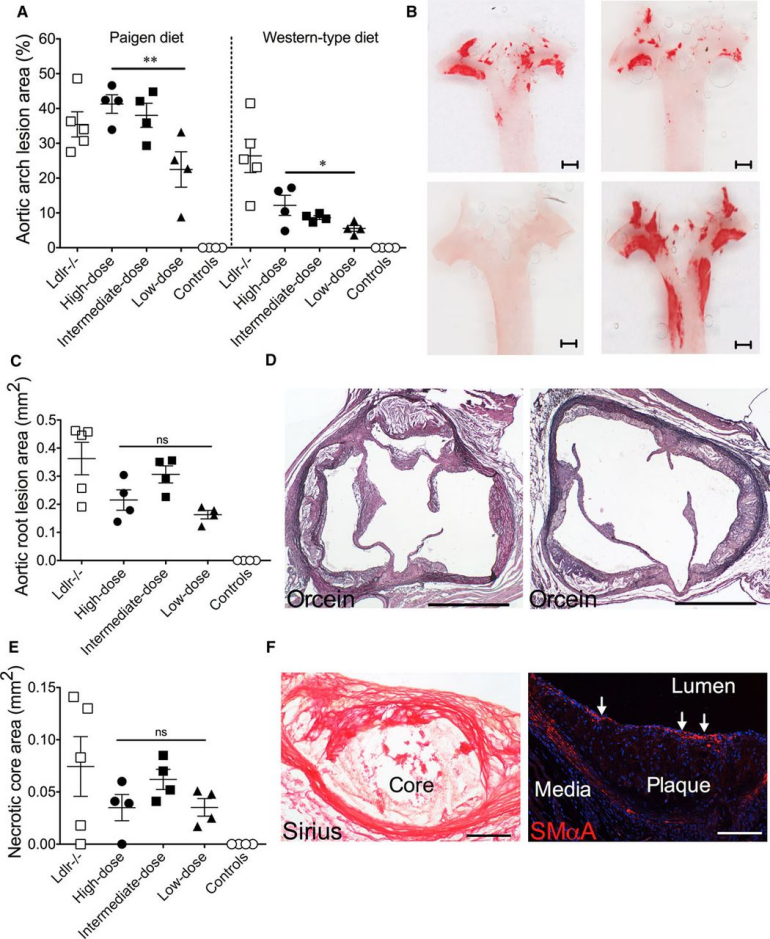
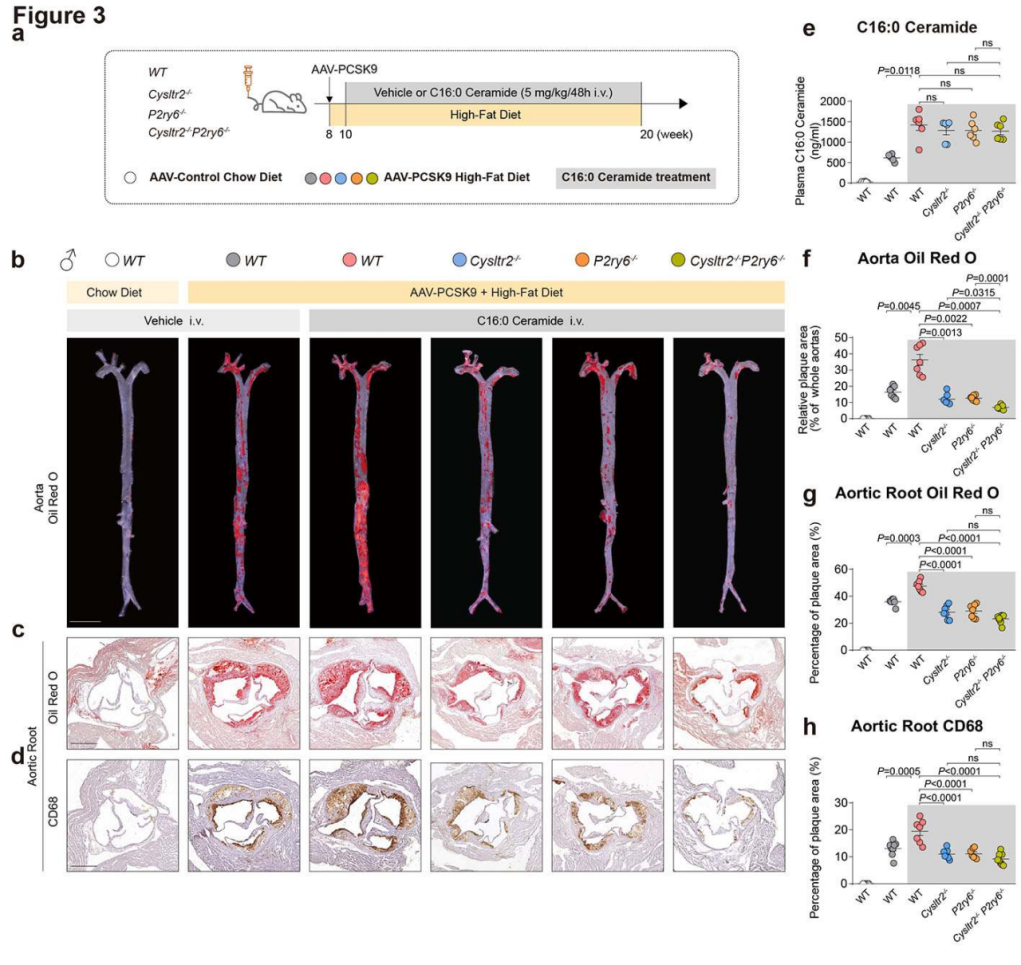
| Product Categories | Product Number | Product Name |
|---|---|---|
| PD Modeling | BC-0997 | rAAV-hSyn-mSNCA-P2A-EGFP |
| BC-1212 | rAAV-hSyn-hSNCA-3xFLAG | |
| BC-2789 | rAVV-CMV-hSNCA(A30P A53T) | |
| BC-1113 | rAAV-CMV-α-synuclein(A30P A53T)-P2A-mCherry | |
| BC-0237 | rAAV-hSyn-hm-α-synuclein(A30P A53T) | |
| AS Modeling | BC-0463 | rAAV-hAAT-mPCSK9(D377Y) |
| BC-1568 | rAAV-hAAT-hPCSK9(D374Y) | |
| AD Modeling | BC-2097 | rAAV-CMV-EGFP-Tau(human) |
| BC-2098 | rAAV-CAG-Tau(human P301L) | |
| BC-0136 | rAAV-EF1a-DIO-Tau(human)-mCherry | |
| BC-0131 | rAAV-CaMKIlα-Tau(Bos mutus)-tdTomato | |
| BC-0133 | rAAV-hSyn-Tau(Bos mutus)-tdTomato |