- E-mail:BD@ebraincase.com
- Tel:+8618971215294
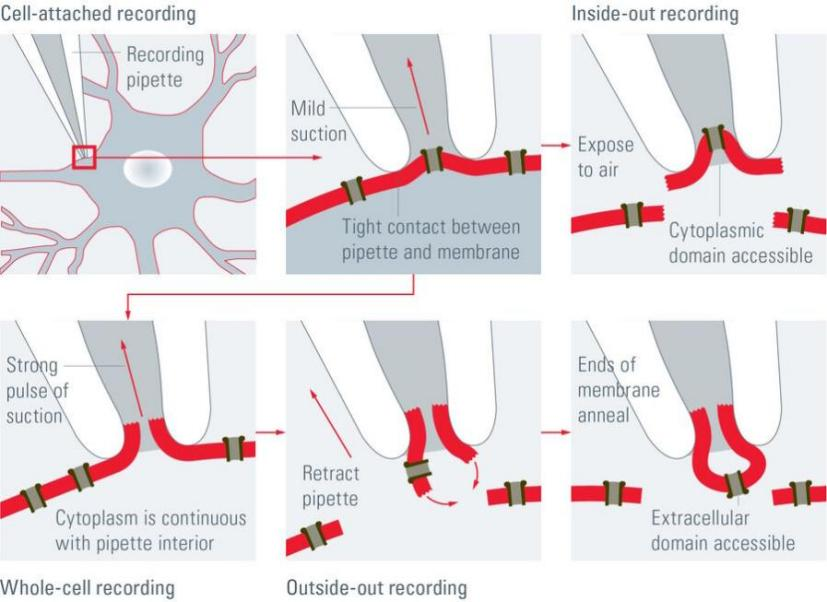
| Detection Object | Recording Target | Indicator | Significance |
|---|---|---|---|
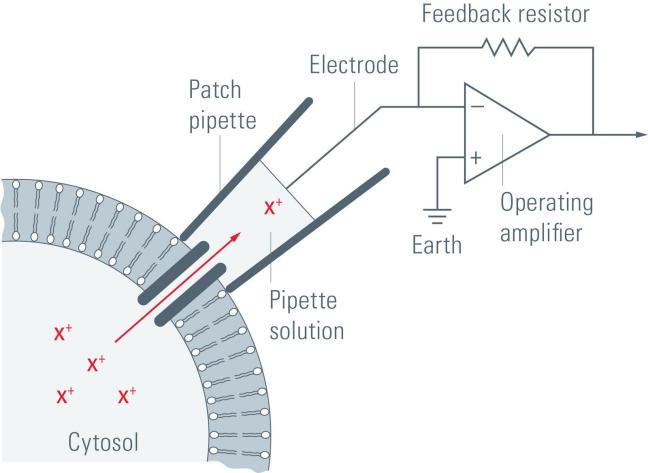
| Brain Slice Patch-Clamp |
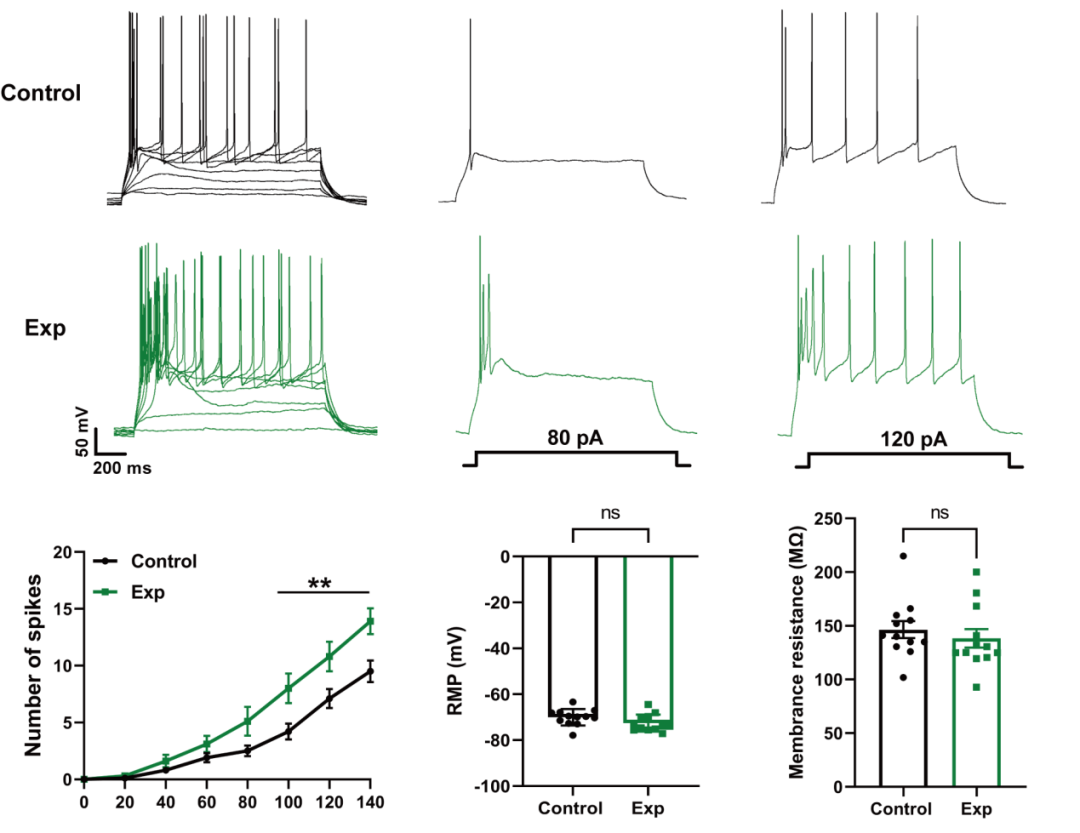
Action potential (AP) | Firing frequency, resting membrane potential (RMP), membrane resistance (Rm), threshold (AP threshold) | Reflects the pattern of neuronal electrical activity |
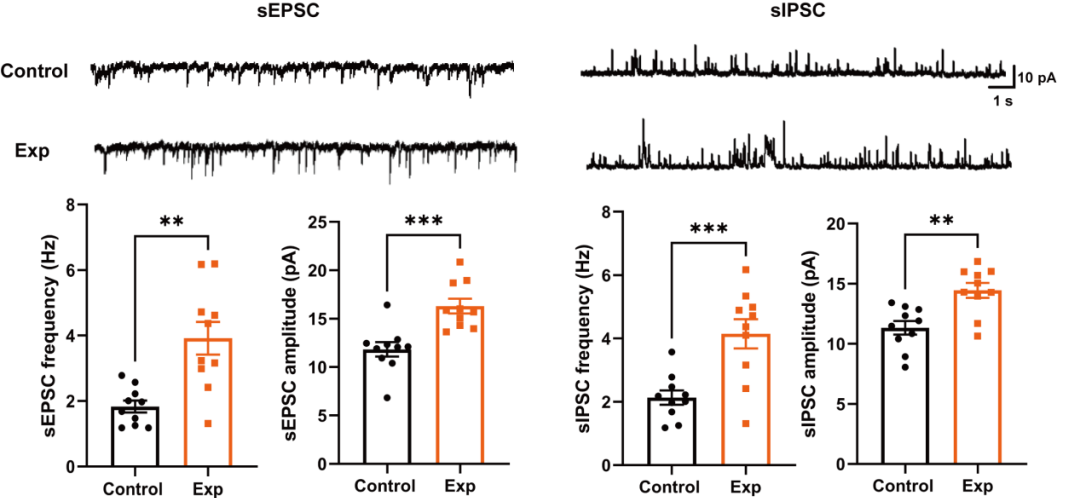
| Miniature excitatory/inhibitory postsynaptic currents (mEPSC/mIPSC), spontaneous excitatory/inhibitory postsynaptic currents (sEPSC/sIPSC) | Frequency, amplitude | Reflects pre- and postsynaptic transmission functions | |
| Long-term potentiation/depression (LTP/LTD) | Slope of field excitatory postsynaptic potential (fEPSC) | Reflects synaptic plasticity related to learning and memory | |
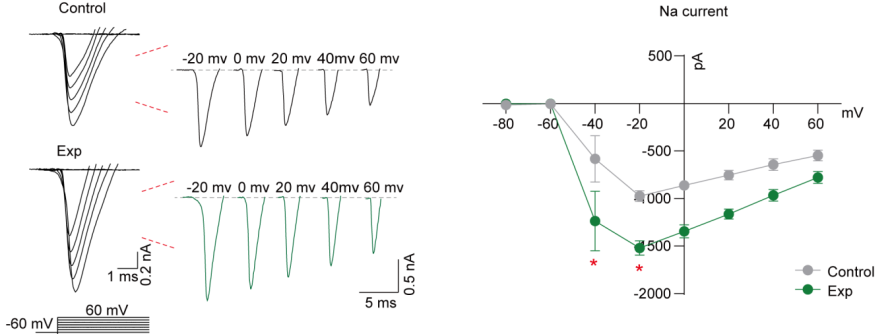
| Cell Patch-Clamp | Ion currents through potassium, sodium, calcium, and other channels | Current density (pA/pF) | Reflects ion channel functions and drug mechanisms of action |