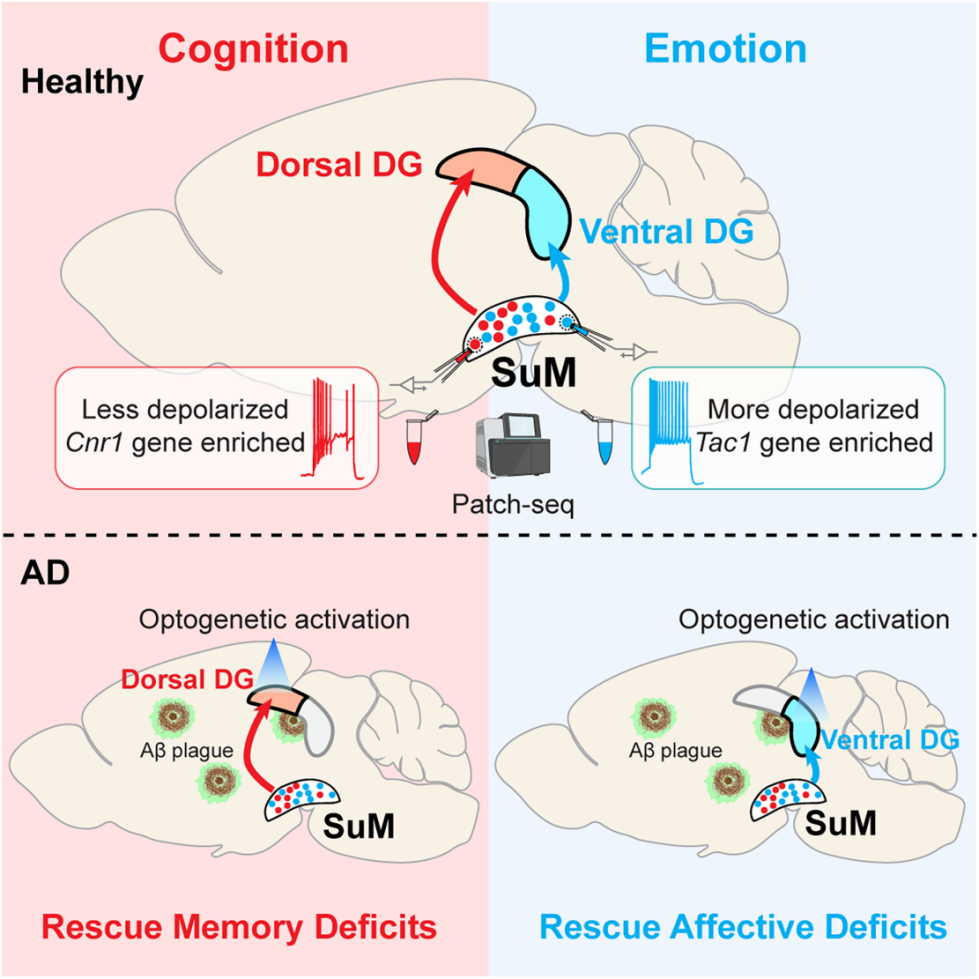
Distinct neuronal subpopulations within the SuM
It is known that the SuM projects primarily to the DG. However, whether SuM neurons projecting to the dDG and ventral vDG constitute distinct subpopulations with unique physiological and molecular features remains unclear.To address this, the authors employed a dual-color monosynaptic retrograde tracing approach using rabies virus (RV). Specifically, they injected RV-eGFP and RV-mCherry into the dDG and vDG, respectively, of Calb1-Cre mice, using DG granule cells (GCs) as starter cells (Figures 1A–1C). The results revealed that SuM-dDG and SuM-vDG neurons were largely non-overlapping, with only 2.78% of labeled cells co-expressing both fluorescent markers (Figures 1D–1G).
To further confirm this anatomical segregation, the authors performed retrograde tracing by injecting cholera toxin subunit B (CTB) conjugated to different fluorophores into the dDG and vDG. Consistent with the RV tracing results, CTB-labeled SuM-dDG (red) and SuM-vDG (green) neurons were intermingled but showed minimal overlap within the SuM, confirming their anatomical distinction.
Next, the authors delivered AAV2-retro-tdTomato or AAV2-retro-eGFP into the dDG or vDG to label projection-specific neurons and recorded their electrophysiological properties in acute brain slices (Figures 1H–I). The results showed clear electrophysiological differences between the two subpopulations: compared with SuM-dDG neurons, SuM-vDG neurons exhibited significantly higher intrinsic excitability (Figures 1J–K) and more depolarized resting membrane potentials (RMPs) (Figure 1L), indicating distinct electrophysiological characteristics between these two groups.
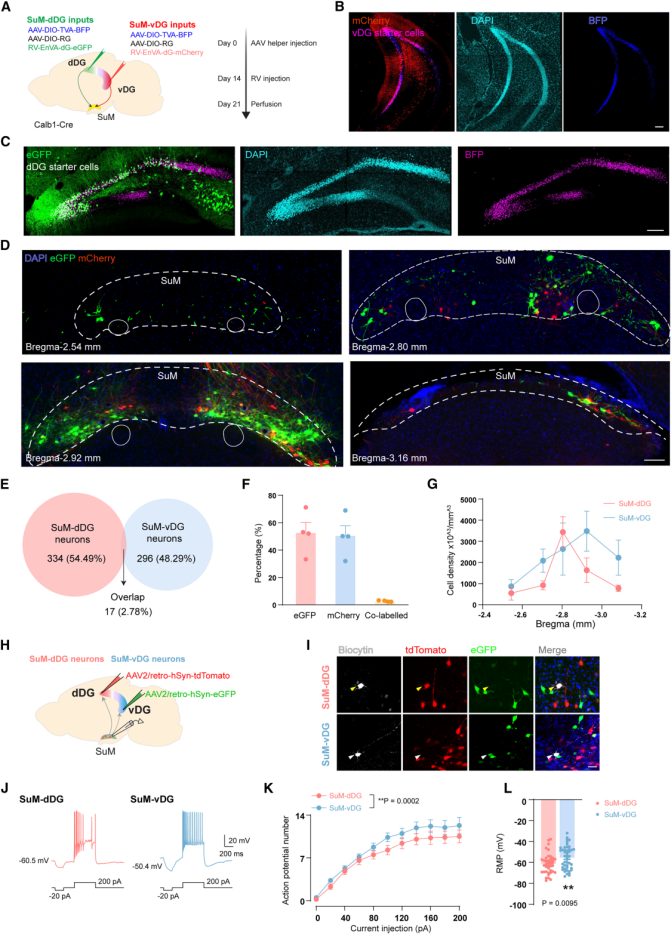 Figure 1. SuM-dDG and SuM-vDG neurons are anatomically segregated and exhibit distinct electrophysiological properties
Figure 1. SuM-dDG and SuM-vDG neurons are anatomically segregated and exhibit distinct electrophysiological properties
To explore the molecular differences between SuM-dDG and SuM-vDG neurons, the authors performed single-nucleus RNA sequencing (snRNA-seq) and patch-seq. After injecting AAV2-retro-eGFP into the dDG or vDG of mice, the SuM was dissected, and nuclei were subjected to snRNA-seq using the 10× Genomics platform (Figure 2A). A total of 19,106 nuclear transcriptomes were captured, and unsupervised clustering identified seven major cell types. Focusing on neurons, transcriptomic profiles further divided them into 12 subclusters (Figures 2B–C), with several subgroups expressing SuM-enriched marker genes.
Because the SuM is small and adjacent to other hypothalamic nuclei, neuronal clusters were mapped onto a high-resolution hypothalamic atlas to accurately identify the core SuM population. Four eGFP-labeled cells from SuM-vDG mice were mapped to SuM core cluster C1 (Figure 2D). Using Seurat (for scRNA-seq/snRNA-seq analysis) to map to a 10× reference dataset, 85.7% of SuM-dDG neurons were assigned to cluster C4, while 83.3% of SuM-vDG neurons were assigned to cluster C1 (Figures 2E–F). Differential expression analysis identified 326 significantly enriched genes (Figure 2I).
Gene set enrichment analysis (GSEA) revealed that SuM-vDG neurons were enriched for pathways related to intrinsic excitability and membrane potential regulation (Figure 2G). Specifically, SuM-dDG neurons highly expressed the excitability-suppressing gene Cnr1, while SuM-vDG neurons highly expressed depolarization-promoting genes such as Scn2a (Figure 2H), consistent with electrophysiological findings showing higher excitability and more depolarized resting membrane potential in SuM-vDG neurons (Figures 1J–1L).
GO analysis further confirmed the functional divergence between the two populations (Figure 2J): SuM-dDG neurons expressed learning and memory–related genes (e.g., Trim67, Cnr1), while SuM-vDG neurons expressed anxiety and depression–related genes (e.g., Tac1) (Figure 2K). Together, these results demonstrate that SuM-dDG and SuM-vDG neurons possess distinct molecular identities.
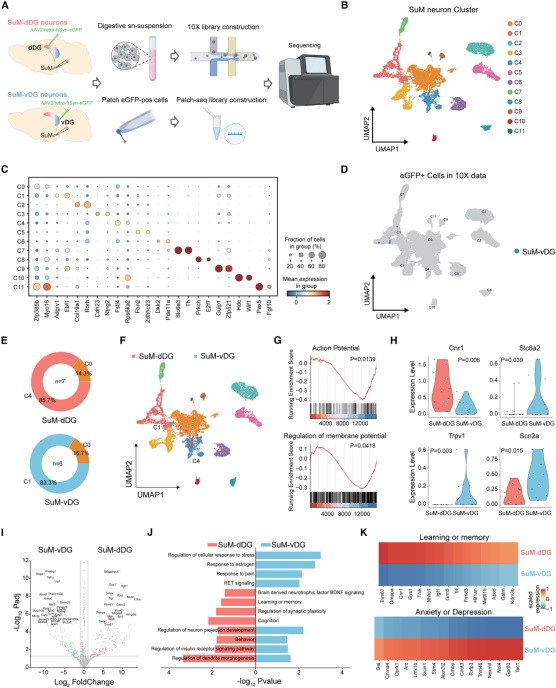
Figure 2. SuM-dDG and SuM-vDG neurons exhibit distinct molecular signatures
Next, the authors examined the input and output connectivity of these two SuM subpopulations. To map monosynaptic inputs, AAV2-retro-Cre was injected into the dDG or vDG, while Cre-dependent helper AAVs encoding TVA and rabies glycoprotein (RG) were injected into the SuM, followed by EnvA-pseudotyped rabies virus (Figure 3A). Results showed that SuM-dDG neurons received more inputs from the lateral hypothalamus (LH), whereas SuM-vDG neurons preferentially received inputs from the posterior hypothalamus (PH) (Figures 3B–3C).
For output mapping, AAV2-retro-Cre was injected into the dDG or vDG, and a Cre-dependent AAV expressing membrane-targeted GFP and presynaptic marker Synaptophysin-mRuby (AAV-FLEX-mGFP-T2A-Synaptophysin-mRuby) was delivered to the SuM (Figure 3D). In this system, green signals labeled axons, red signals labeled presynaptic terminals, and only overlapping (yellow) signals were quantified to minimize false positives.
Whole-brain quantification revealed that each SuM subpopulation predominantly targeted its corresponding DG region (Figures 3E–F): SuM-dDG neurons formed substantially more synapses with the dDG, while SuM-vDG neurons preferentially innervated the vDG. Both subpopulations projected sparsely to hippocampal CA1/2/3 regions, and no significant differences were observed in projections to extrahippocampal regions such as the medial septum (MS) and horizontal diagonal band (HDB), indicating projection selectivity.
In summary, through snRNA-seq, patch-seq, rabies virus tracing, and projection mapping, the study confirmed that SuM-dDG and SuM-vDG neurons represent molecularly and functionally distinct subpopulations that differ in electrophysiological properties, gene expression, and input–output connectivity patterns.
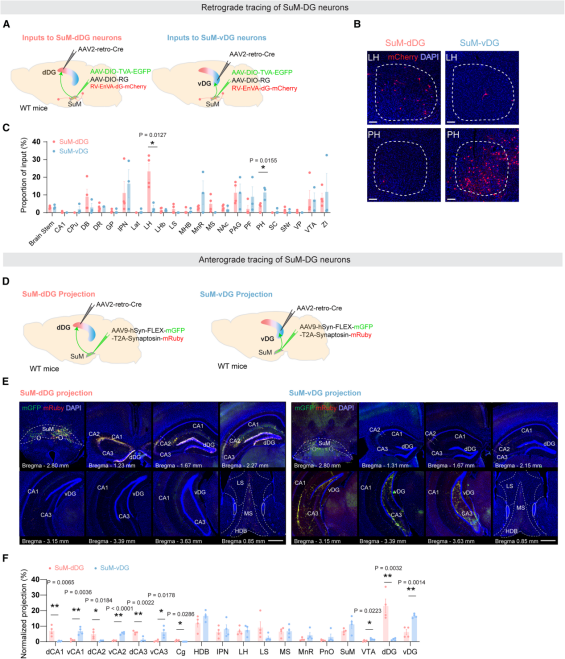 Figure 3. SuM-dDG and SuM-vDG neurons display distinct input and output connectivity patterns
Figure 3. SuM-dDG and SuM-vDG neurons display distinct input and output connectivity patterns
Distinct involvement of SuM subpopulations in regulating cognitive and emotional functions
To determine whether SuM-dDG and SuM-vDG neurons are differentially involved in cognitive and emotional regulation, the authors employed fiber photometry to record calcium dynamics in these two SuM subpopulations expressing GCaMP6f during hippocampus-dependent behavioral tasks (Figure 4A). Two representative paradigms were used: the Novel Place Recognition (NPR) test, which assesses spatial memory, and the Tail Suspension Test (TST), which evaluates depression-like behavior.
During the NPR task, SuM-dDG neurons showed significantly increased activity during the memory retrieval phase compared with the encoding phase (Figures 4B–4I, 4M). In contrast, during the TST, SuM-vDG neurons displayed a marked increase in calcium activity when mice transitioned from immobility to movement (Figures 4J–4L, 4N). These findings indicate that SuM-dDG and SuM-vDG neurons are differentially engaged in modulating cognitive and emotional functions, respectively.
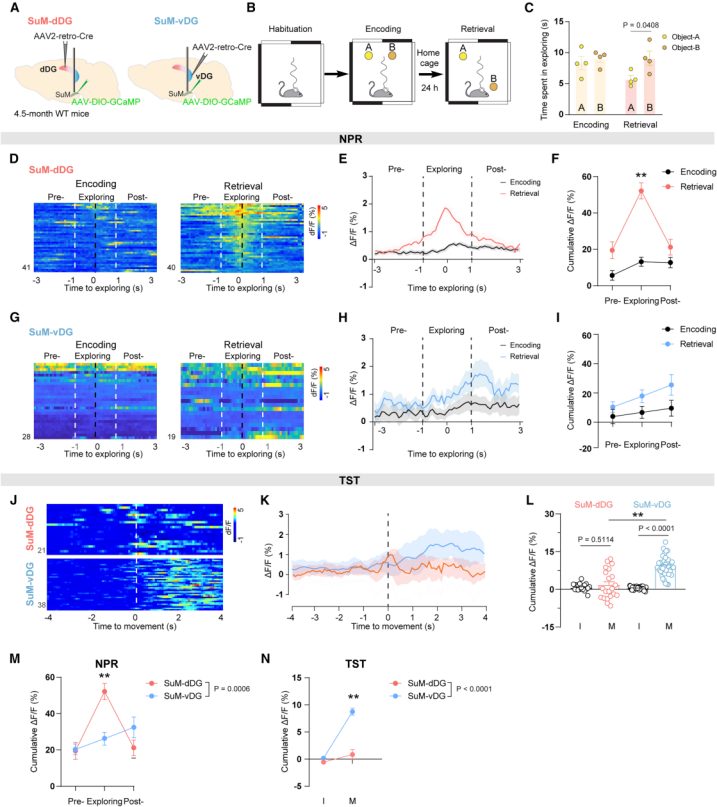 Figure 4. Distinct SuM subpopulations are differentially involved in cognitive and emotional function regulation
Figure 4. Distinct SuM subpopulations are differentially involved in cognitive and emotional function regulation
Distinct SuM subpopulations are selectively required for cognitive and emotional behaviors
After establishing the unique roles of SuM-dDG and SuM-vDG neurons in cognition and emotion, the authors used a chemogenetic approach to selectively inhibit each subpopulation (Figures 5A, 5D), and verified the effectiveness of inhibition via c-Fos immunostaining (Figures 5B–5C, 5E–5F). When SuM-dDG neurons were inhibited, mice exhibited reduced discrimination index in the Novel Place Recognition (NPR) test (Figures 5G–5H) and decreased freezing time in Contextual Fear Conditioning (CFC) training (Figures 5I–J), while locomotor activity remained unaffected (Figure 5L), indicating impaired memory performance.
In contrast, inhibition of SuM-vDG neurons led to reduced time spent in the center of the Open Field Test (Figures 5K–5M), reduced time in the open arms of the Elevated Zero Maze (Figures 5N–5O), and increased immobility time in the Tail Suspension Test (TST) (Figure 5P), suggesting exacerbated anxiety- and depression-like behaviors. Together, these results demonstrate that SuM-dDG neuronal activity is selectively required for cognitive functions, while SuM-vDG neuronal activity is selectively required for emotional regulation.
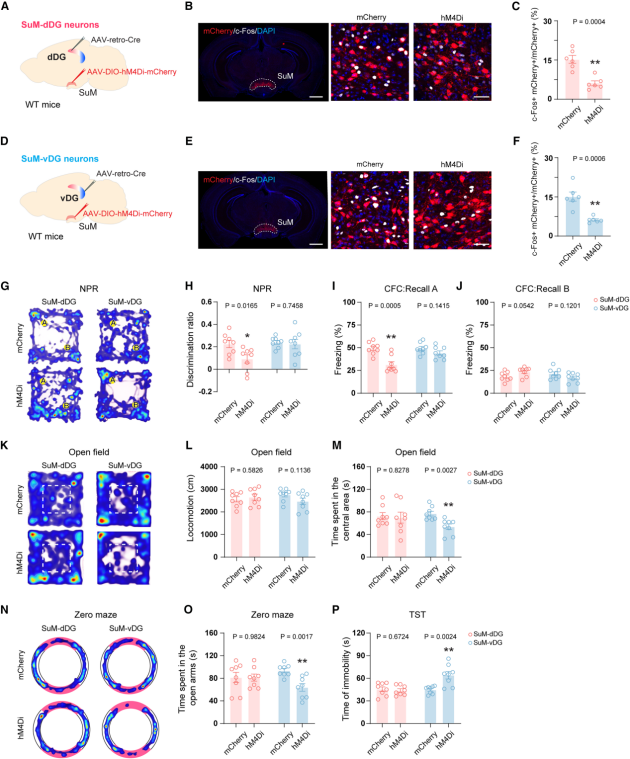
Figure 5. Distinct SuM subpopulations are selectively required for cognitive and emotional behaviors
SuM-dDG and SuM-vDG pathways selectively regulate cognitive and emotional behaviors
To determine whether activation of these two SuM pathways is sufficient to modulate cognitive and emotional functions, the authors employed optogenetic stimulation of SuM projections. An AAV expressing CaMKII-ChR2-YFP was delivered to the SuM, and optical fibers were implanted above the dDG or vDG to specifically activate the corresponding pathways during behavioral tests (Figures 6A–B).
In cognitive behavioral assays, activation of the SuM-dDG pathway during the memory retrieval phase enhanced cognitive performance: mice showed an increased discrimination index in the NPR test (Figure 6C) and prolonged freezing time in the CFC test at both 24 hours and 7 days (with no change in context B; Figures 6D–6F).
In emotional behavioral tests, activation of the SuM-vDG pathway produced anxiolytic and antidepressant-like effects: mice spent more time in the open arms of the Elevated Zero Maze (EZM) (Figure 6I), exhibited decreased immobility in the TST and Forced Swim Test (FST) (Figures 6J–K), and showed increased sucrose preference in the Sucrose Preference Test (SPT) (Figure 6L), indicating alleviation of anxiety-, depression-, and anhedonia-like behaviors. Notably, activation of either pathway did not alter locomotor activity or the time spent in the center zone of the Open Field Test (Figures 6G–H). Together, these results demonstrate that the SuM-dDG and SuM-vDG pathways selectively regulate cognitive and emotional behaviors, respectively.
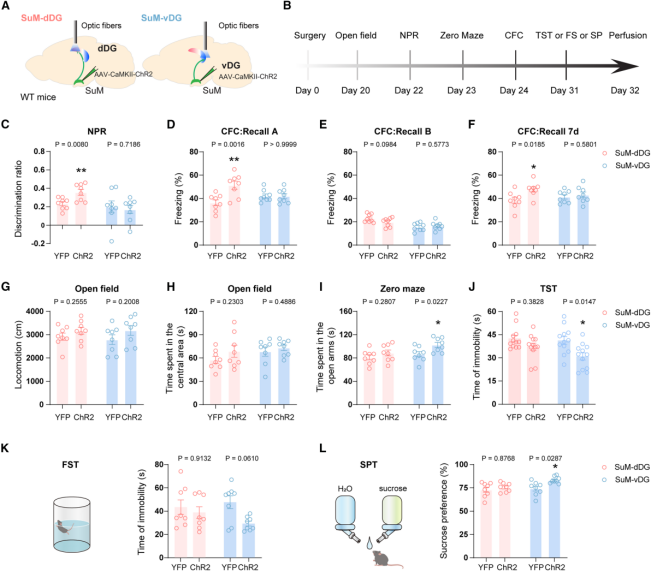 Figure 6. Optogenetic activation of SuM-dDG or SuM-vDG projections selectively modulates memory and emotional behaviors
Figure 6. Optogenetic activation of SuM-dDG or SuM-vDG projections selectively modulates memory and emotional behaviors
DG-projecting SuM neurons exhibit aberrant activity in AD mice during behavioral tasks
To evaluate in vivo neuronal activity, the authors performed fiber photometry recordings of calcium dynamics in SuM-dDG and SuM-vDG neurons. Under baseline conditions (in the home cage), both SuM-dDG and SuM-vDG neurons in 5×FAD mice (an Alzheimer’s disease model) showed significantly reduced calcium activity compared with wild-type (WT) mice (Figures 7A–7J).
To further assess task-specific abnormalities in AD mice, calcium activity was monitored during the NPR task (Figure 7A) and the TST (Figure 7B). During the NPR retrieval phase, SuM-dDG activity in AD mice was markedly lower than in WT mice (Figures 7K–7M), while SuM-vDG activity remained unchanged (Figures 7N–7P). In contrast, during the TST, SuM-vDG neurons in AD mice showed a blunted increase in activity during transitions from immobility to movement (Figures 7Q–7S)—a pattern associated with depression-like behavior—while SuM-dDG activity showed no abnormalities (Figures 7T–7V).
Together, these results indicate that in AD model mice, SuM-dDG and SuM-vDG neurons exhibit intrinsic functional impairments and reduced baseline activity, along with distinct patterns of task-related dysfunction.
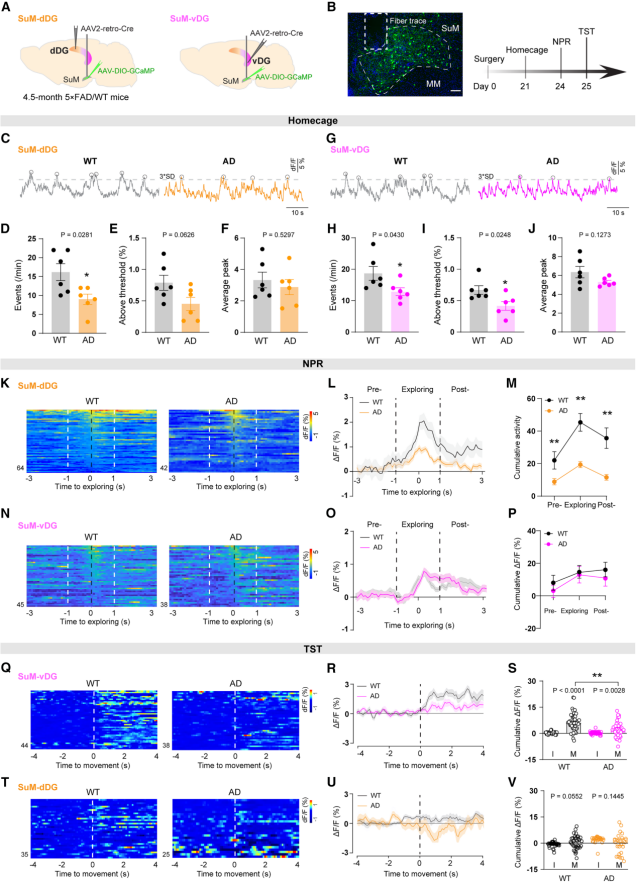 Figure 7. DG-projecting SuM neurons show aberrant activity in AD mice
Figure 7. DG-projecting SuM neurons show aberrant activity in AD mice
Optogenetic activation of the SuM–dDG or SuM–vDG pathways selectively improves cognitive and emotional deficits in AD model mice
Since SuM–DG neuronal baseline and behaviorally induced activities were reduced in AD model mice, the authors applied an optogenetic strategy similar to that used in WT mice to activate the SuM–dDG/SuM–vDG pathways during behavioral testing (Fig. 8A–B) to explore potential therapeutic effects.
Activation of the SuM–dDG pathway (during the memory retrieval phase) increased the discrimination index in the NPR test (Fig. 8C), and restored freezing time in context A (but not B) of the CFC test to WT levels (Fig. 8D–8F), indicating memory improvement. Meanwhile, no changes were observed in the OFT, EPM, TST, FST, or SPT (Fig. 8G–8L), suggesting a selective rescue of memory deficits.
In contrast, activation of the SuM–vDG pathway increased time spent in the open arms in the EPM (Fig. 8I), reduced immobility time in the TST and FST, and restored sucrose preference to WT levels in the SPT (Fig. 8J–8L), indicating relief of anxiety- and depression-like behaviors. These changes occurred without affecting memory performance (Fig. 8C–8F) or locomotor activity and center time in the OFT (Fig. 8G–H), suggesting a selective improvement in emotional deficits. In summary, optogenetic activation of the SuM–dDG and SuM–vDG pathways selectively rescues cognitive and emotional impairments, respectively, in AD model mice.
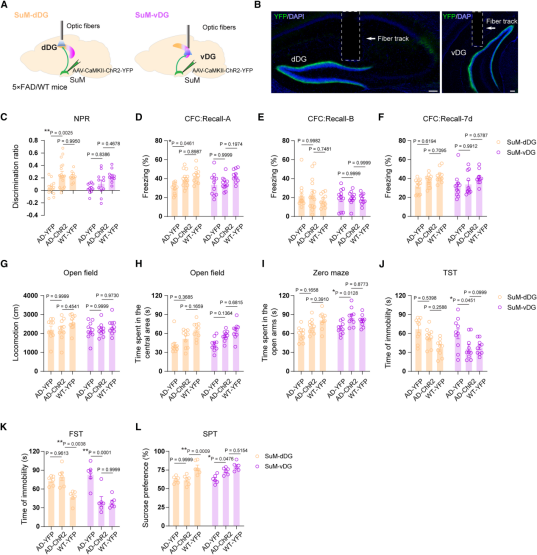
Figure 8. Optogenetic activation of the SuM–dDG/vDG pathways selectively rescues cognitive and emotional deficits in AD mice.
Summary
This study provides the first evidence that SuM–DG subcircuits independently and in parallel regulate cognitive and emotional functions, revealing the circuit mechanisms underlying multiple AD symptoms and challenging the conventional notion of “a single pathway controlling multiple symptoms.” Since the SuM shows no AD pathological deposition, it represents an ideal therapeutic target. Targeted activation of the SuM–dDG or SuM–vDG pathways may selectively improve cognitive or emotional deficits in AD patients.
The viral tools used in this study were provided by Brain Case Biotech as follows: