- E-mail:BD@ebraincase.com
- Tel:+8618971215294

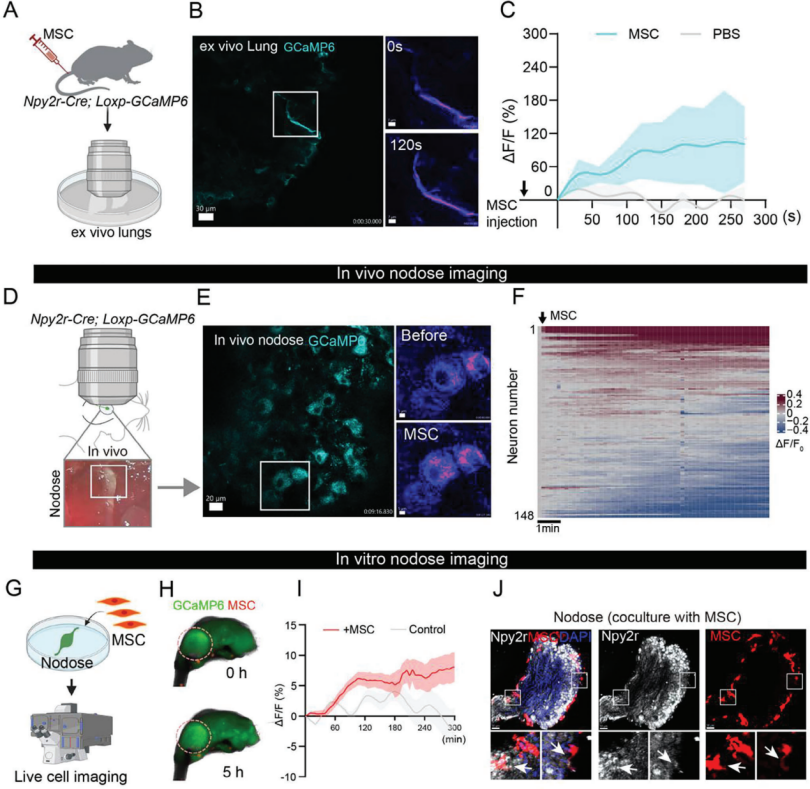
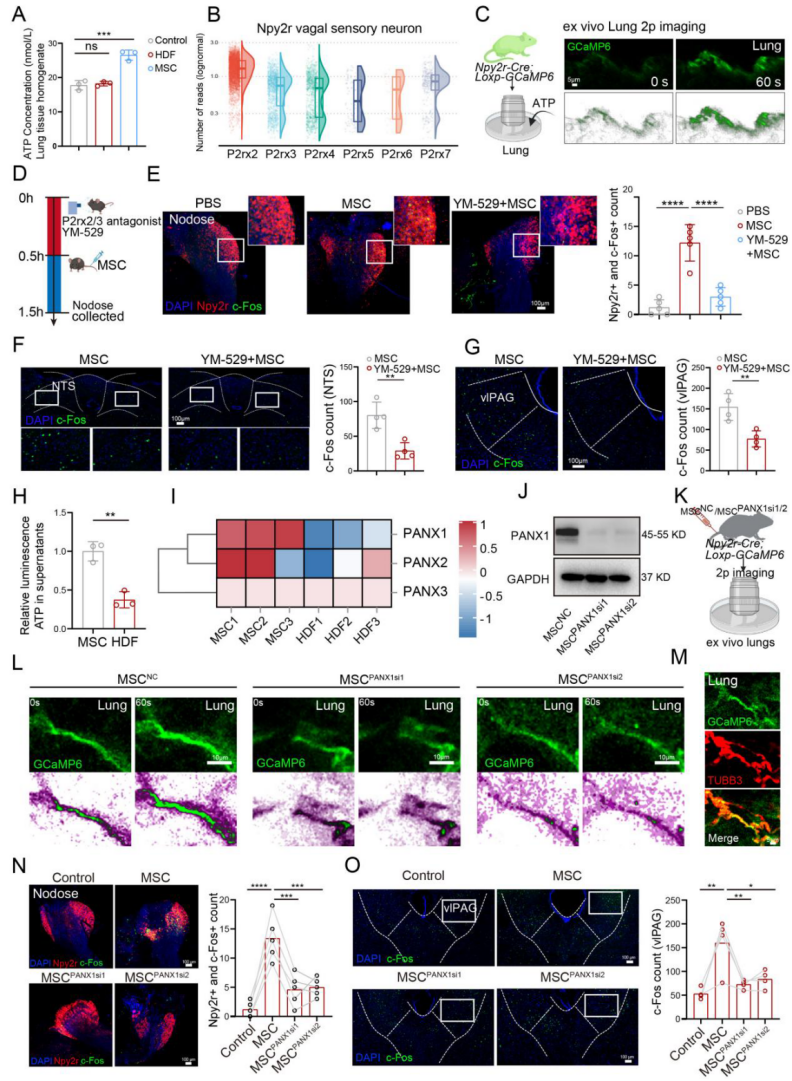
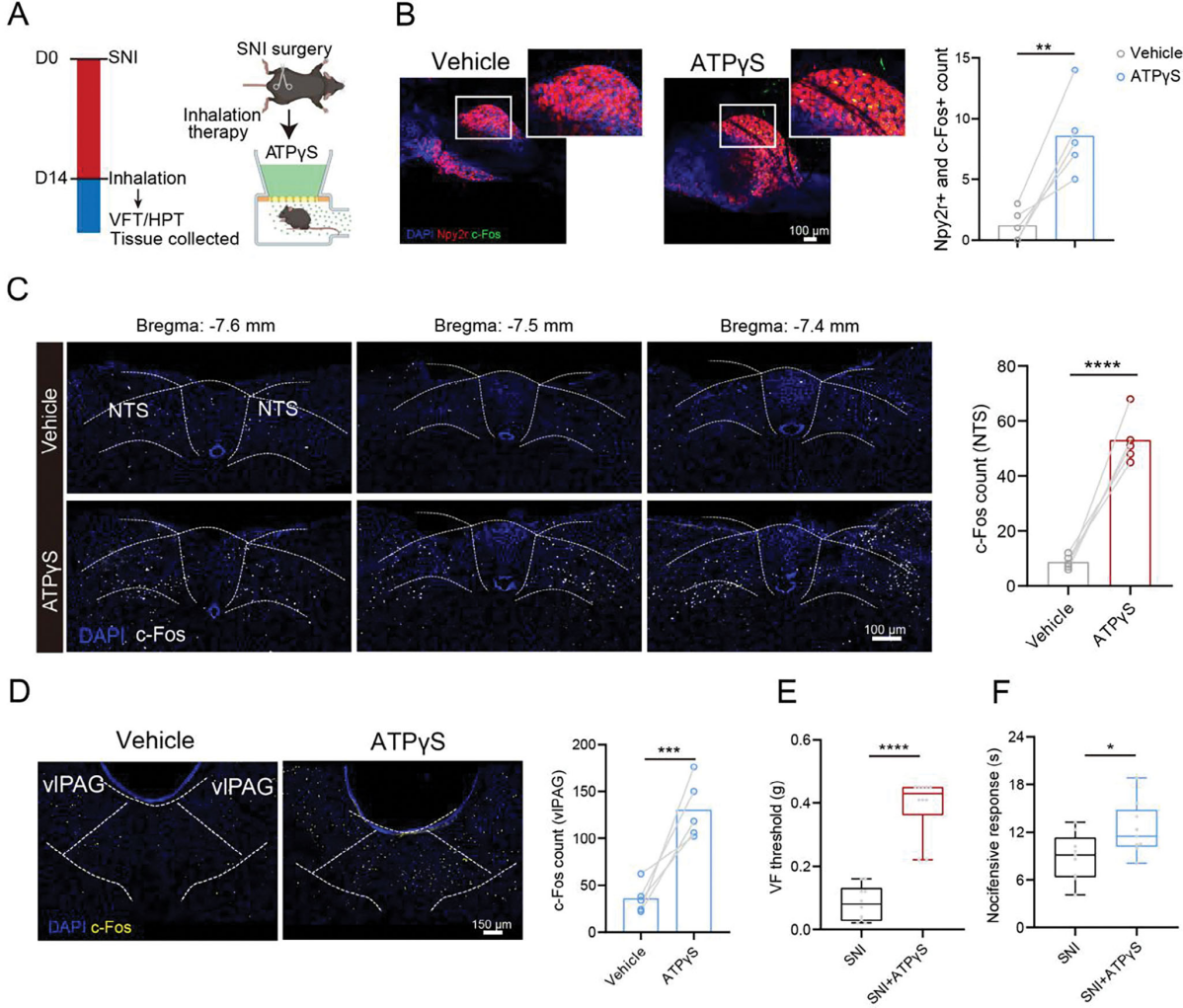
| Product No. | Product Name |
|---|---|
| BC-0653 | rAAV-hSyn-DIO-taCasp3-T2A-TEVp |
| BC-0015 | rAAV2-retro-DIO-EGFP |
| BC-0016 | rAAV2-retro-DIO-mCherry |
| BC-0623 | rAAV2-retro-DIO-hM4D(Gi)-EGFP |
| BC-0143 | rAAV2-retro-DIO-hM3D(Gq)-mCherry |
| BC-0238 | rAAV-retro-DIO-GCaMP6s |
| BC-0077 | rAAV-hSyn-GCaMP6s |
| BC-PRV-801 | PRV-CAG-3Gc |
| BC-HSV-HBEGFP | H129-hUbC-HBEGFP |