- E-mail:BD@ebraincase.com
- Tel:+8618971215294
In Alzheimer's disease (AD) patients, the hippocampus is one of the first regions to be affected. Episodic memory loss is a prominent clinical feature of AD and is closely associated with tau pathology and hippocampal damage. But how do different hippocampal neurons respond to tau accumulation, and what roles do they play in AD-like social memory loss? This study elucidates distinct protein and phosphorylation networks between dCA1 and vCA1, highlighting the susceptibility of vCA1 microcircuits to AD-like tau accumulation.
In 6-month-old male 3xTg-AD mice, a significant number of AT8-ir neurons were observed in the vCA1 region, but not in the dCA1. Similarly, in 3-month-old male and female P301L mice expressing the longest hTau isoform and the FTDP-17 P301L mutation, hyperphosphorylated tau significantly accumulated in the cell bodies and neuronal processes of the ventral hippocampus, particularly in the vCA1 region. These data suggest that the vCA1 is more sensitive to AD-like tau pathology than the dCA1.
To further investigate the differences between the vCA1 and dCA1 under physiological conditions and in the presence of AD-like tau pathology, researchers performed proteomic and phosphoproteomic analyses in WT and P301L mice. They found 158 differentially expressed proteins between the vCA1 and dCA1 in WT mice, indicating distinct protein networks along the anterior-posterior axis of the hippocampal CA1 region under physiological conditions. Compared to WT mice, P301L mice had 50 and 45 differentially expressed proteins in the vCA1 and dCA1, respectively. In the vCA1, the top 30 differentially expressed proteins between WT and P301L mice were different from those in the dCA1.
Subsequently, they conducted phosphoproteomic analysis to delineate the distinct phosphoprotein networks in the two groups. Compared to the dCA1, the vCA1 in WT and P301L mice showed modifications in 644 and 505 proteins and 1021 and 839 sites, respectively (For details, see the original text in Supplementary File 1: Fig. S6). In the vCA1 of P301L mice, the phosphorylation levels of tau at T181 and S191 (tau-T181 and tau-S191) and CaMKIIa at S272 (CaMKIIa-S272) were significantly higher than in the vCA1 of WT mice (Fig. 1d). However, the phosphorylation level of neuronal navigator 1 at S197 (NAV1-S197) was lower in the vCA1 of P301L mice than in WT mice (Fig. 1d). These data indicate that the distribution of AD-like tau pathology-related protein and phosphoprotein networks differs along the anterior-posterior axis of the hippocampal CA1 region.TauvCA1 accumulation impairs social memory.
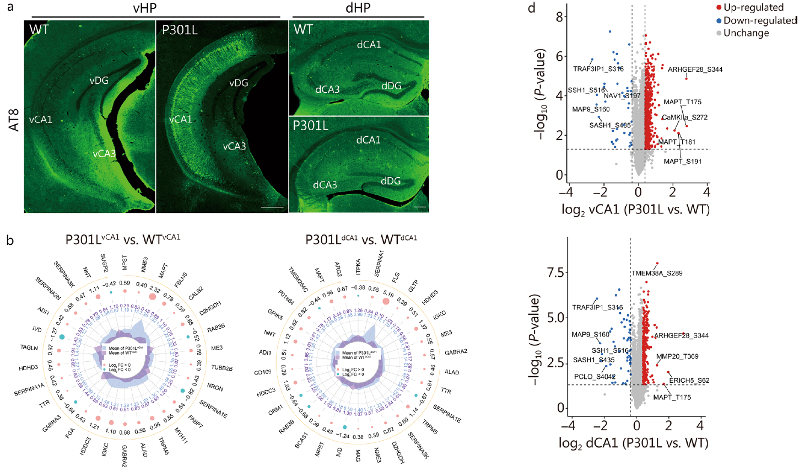
Fig. 1 Proteomics and phosphoproteomics of dorsal hippocampal CA1 (dCA1) and ventral hippocampal CA1 (vCA1) in P301L mice.
Researchers conducted social exploration and memory tests on 6-month-old 3xTg-AD mice that showed significant p-Tau accumulation in the vCA1. During exploration, both nTg and 3xTg-AD mice spent more time on the side with a new mouse than on the side with an empty cup, indicating a preference for social approach (P < 0.01). Notably, nTg mice showed a significant preference for the new stimulus in the social memory test (P < 0.01), while this preference was not observed in 3xTg-AD mice.
Subsequently, researchers performed social exploration and memory tests on 3-month-old male P301L mice, which accumulated hyperphosphorylated tau in the vCA1 but not in the dCA1. In the social tests, both WT and P301L mice showed a strong preference for social approach (P < 0.01), but P301L mice spent equal time interacting with familiar and new individuals (P > 0.05) and had lower social recognition scores (P < 0.01). These findings suggest that pathological tau accumulation in the vCA1 is associated with social memory deficits in AD mice.
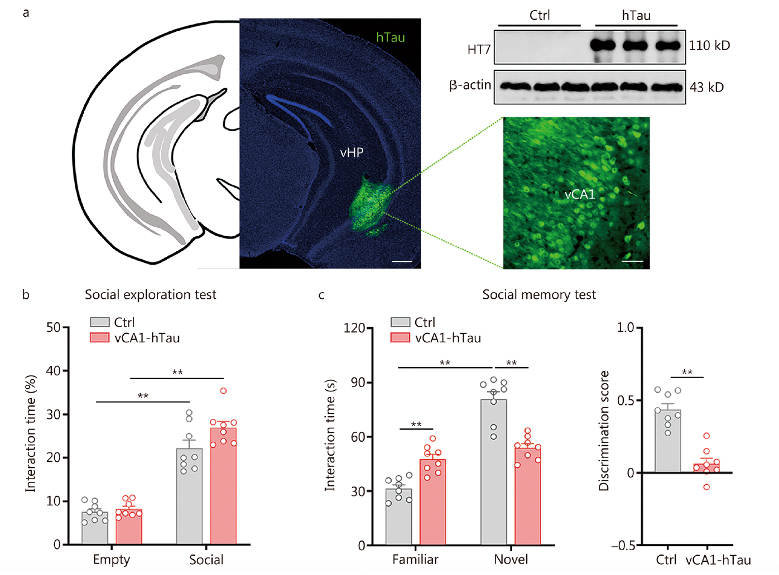
Fig. 2 Tau accumulation in vCA1 impairs social memory, For more details, see the original figures in the supplementary material of the text.
To investigate which types of hippocampal neurons are sensitive to the accumulation of pathological tau and responsible for tau-induced social memory deficits, researchers performed double immunostaining on brain slices from nTg and 3xTg-AD mice containing the vCA1 subregion. Compared to nTg mice, more AT8 and pT205 signals were colocalized with CaMKII (a marker of excitatory neurons) and PV (a marker of PV neurons), respectively. Quantitative analysis of 6-month-old 3xTg-AD mice showed that 84% of AT8+ signals were located within CaMKII neurons, and 92% of PV neurons were pT205-ir. These data indicate that both excitatory and PV neurons in the vCA1 are prone to AD-like tau accumulation.
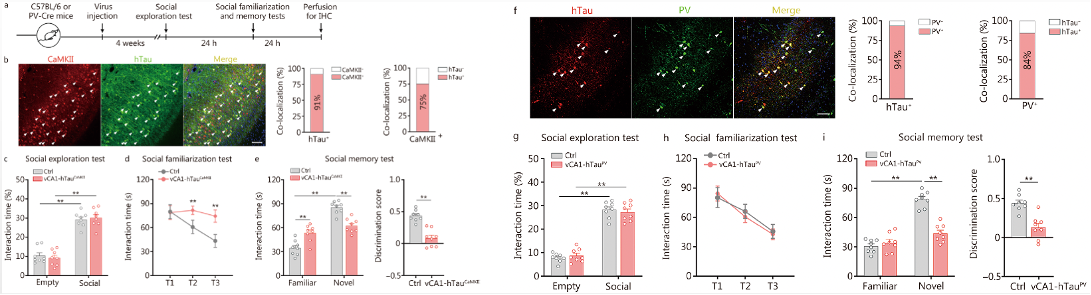
Fig. 3 Overexpression of hTau in excitatory and PV neurons impairs social memory
The researchers assessed the changes in excitability of excitatory and PV neurons in the vCA1 after overexpressing hTau using in vitro patch-clamp recordings. They also analyzed the real-time activity of excitatory and PV neurons in the vCA1 during social recognition via in vivo calcium imaging recordings. Given that PV neurons inhibit local excitatory neurons through the neurotransmitter GABA, the researchers further investigated the changes in GABA release to excitatory neurons in vCA1-hTauPV mice and its contribution to social memory deficits. They used AAV-CaMKII-iGABASnFR (a genetically encoded GABA sensor obtained from Brain Case Biotech) to target excitatory neurons in the vCA1 and monitored real-time GABA signals in vCA1-hTauPV mice throughout the social memory test. They found that hTau accumulation inhibits and disrupts the discharge of excitatory and PV neurons in the vCA1. The accumulation of hTau in excitatory neurons impairs social memory by suppressing the discharge of excitatory neurons associated with familiar conspecific recognition. In contrast, hTau accumulation in PV neurons leads to the misidentification of a novel identical familiar feature due to the lack of inhibition in the excitatory neurons of the vCA1.
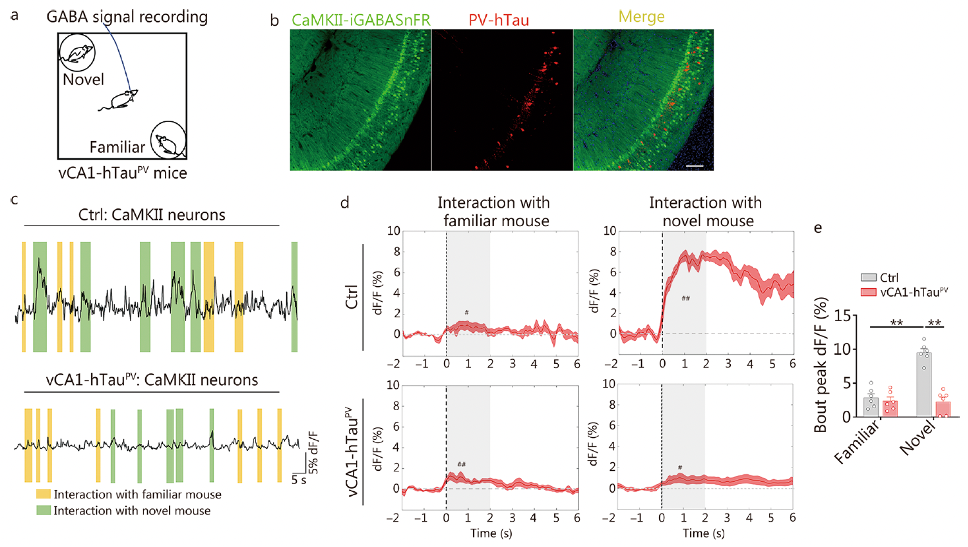
Fig. 4 vCA1-hTauPV accumulation disinhibits excitatory neurons during novel conspecific identification
Using genetically encoded optogenetic tools to activate excitatory neurons and PV neurons in the vCA1 region significantly increased the interaction time and recognition scores with novel conspecifics in mice. This indicates that optogenetic activation of excitatory neurons and PV neurons can alleviate and correct social memory deficits caused by hTau accumulation.
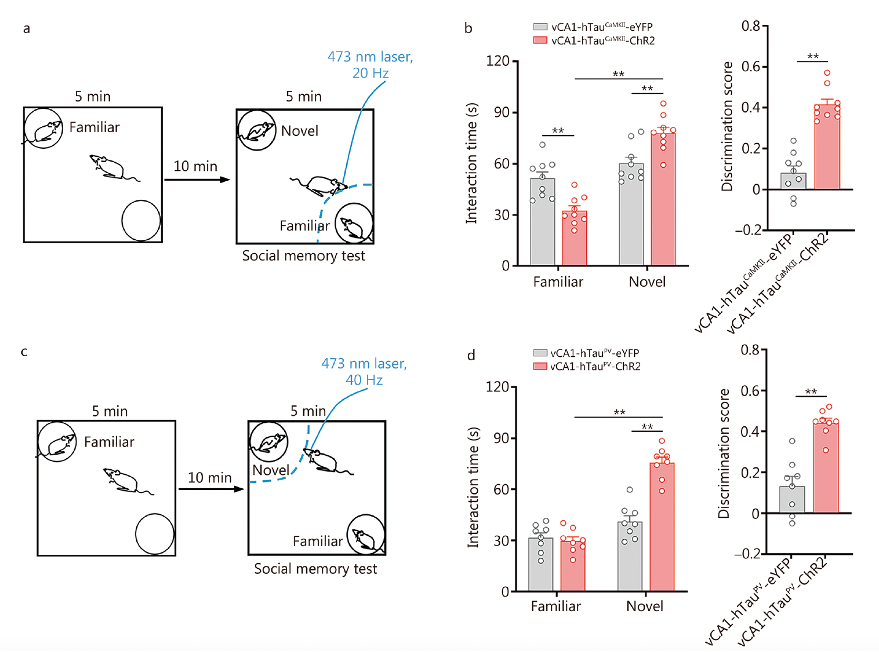
Fig. 5 Photoactivation of excitatory and PV neurons rescues tau accumulation-impaired discrimination in social memory.
At specific concentrations, the natural triterpenoid compound ursolic acid (UA) significantly reduced total Tau (tau5, HT7) and phosphorylated Tau (p-Tau; AT8) levels in primary neurons and HEK293 cells expressing full-length hTau. In vivo, vCA1-hTau mice treated with low-dose UA [15 mg/(kg·d)] exhibited a significant decrease in total Tau, hTau, and p-Tau levels (P < 0.05 or P < 0.01; Additional file 1: Fig. S26a). This treatment also improved social memory, as evidenced by increased exploration of novel mice and higher recognition scores (P < 0.01; Additional file 1: Fig. S26b, c). No adverse effects were observed in C57BL/6 mice treated with low-dose UA (P > 0.05; Additional file 1: Fig. S27). UA treatment promoted autophagy by enhancing the translocation of TFEB from the cytoplasm to the nucleus (Fig. 6b), increased LC3B-II levels, and decreased p62 levels in HEK293-hTau cells. The beneficial effects of UA on tau reduction, enhanced autophagy, recovery of neuronal excitability, and improvement in social memory were abolished by knocking down TFEB using AAV-hSyn-shTFEB (P < 0.01; Fig. 6e-j, Additional file 1: Fig. S28). Therefore, UA can rescue social memory deficits caused by tau accumulation through TFEB-dependent autophagy enhancement, potentially offering a promising strategy for preventing AD.
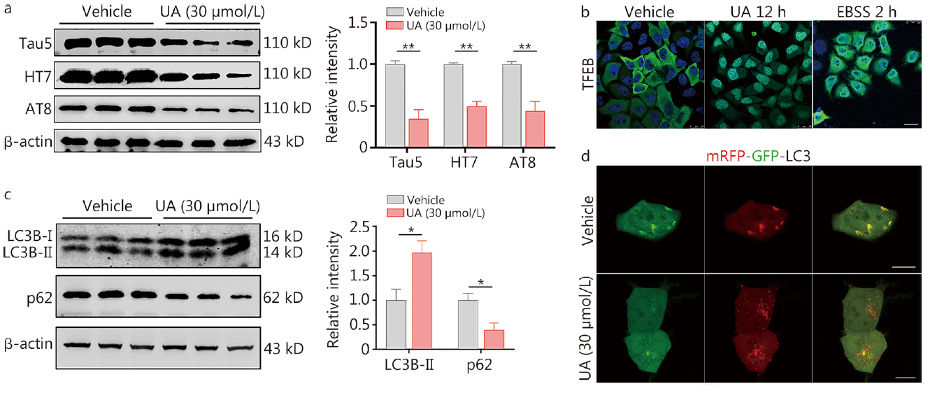
Fig. 6 Ursolic acid (UA) reduces the pathological tau load to improve social memory via TFEB(For details on the results of downregulating TFEB protein expression induced by UA, see the original images in Figure 7 e-j of the text)
In this article, the researchers investigated the changes in GABA release to excitatory neurons in vCA1-hTauPV mice and its contribution to social memory deficits. They used our company's genetically encoded GABA sensor “BC-0341 AAV-CaMKII-iGABASnFR.”
If you are interested in the application of this product, please feel free to contact us at bd@braincase.cn.
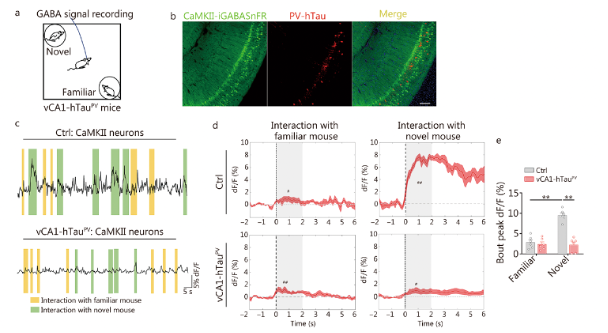
Fig. 7 Schematic of GABA signal recording in the social memory test. Detection of GABA neurotransmitter signaling.