- E-mail:BD@ebraincase.com
- Tel:+8618971215294
The interactions between microglia and neurons play a crucial role in the regulation of various physiological and behavioral functions. Researchers have reported that acute restraint stress leads to increased activity of GABAergic neurons (GABACeA) in the central amygdala (CeA) of male mice, resulting in anxiety-like behaviors within 12 hours. Meanwhile, the MST4-NF-κB-CX3CL1 signaling pathway in GABACeA neurons mediates the activation of microglia. Activated microglia, in turn, inhibit the activity of GABACeA neurons by engulfing their dendritic spines, ultimately leading to the extinction of anxiety-like behaviors induced by restraint stress. These findings reveal a dynamic molecular and cellular mechanism by which microglia drive negative feedback to inhibit GABACeA neuronal activity, thereby maintaining brain homeostasis in response to acute stress.
Researchers induced anxiety-like behaviors in male mice through acute restraint stress (ARS-2h) for 2 hours. Using the open field test (OFT) and elevated plus maze (EPM) test, they found that ARS-2h mice exhibited significant changes in exploratory behaviors at 0.5 hours, 4 hours, and 8 hours post-stress induction, which disappeared within 12 hours. By using optogenetic tagging in the CeA nuclei of GAD2-Cre mice, the researchers discovered that the spontaneous spike firing rate of GABACeA neurons in ARS-2h mice increased, but gradually decreased over time, returning to levels comparable to the unstressed control group by 12 hours post-restraint. This was consistent with the behavioral test results. To further investigate how GABACeA neurons participate in exploratory behaviors, the researchers inhibited GABACeA neuron activity by locally injecting AAV-DIO-hM4Di-mCherry into the bilateral CeA of GAD2-Cre mice. Whole-cell patch-clamp recordings in acute brain slices revealed that the resting membrane potential (Vrest) of GABACeA neurons was hyperpolarized after perfusion with Clozapine-N-oxide (CNO, 10 μM). Additionally, in ARS-2h mice, chemogenetic inhibition of GABACeA neurons increased the time spent in the central area of the OFT and the number of entries into the open arms of the EPM.
These results indicate that acute restraint stress increases GABACeA neuronal activity and induces anxiety-like behaviors in mice, while a decrease in this neuronal activity is accompanied by the extinction of anxiety-like behaviors.
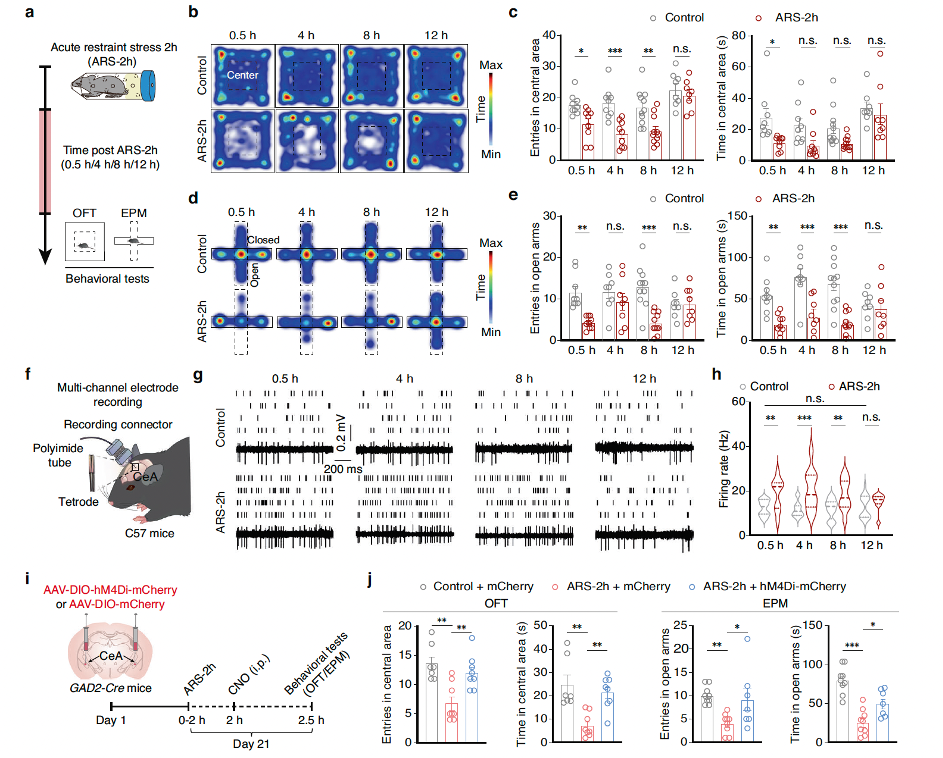
Fig1. Through behavioral analysis, optogenetic labeling, and chemogenetic manipulation, it was verified that acute restraint stress increases GABACeA neuronal activity and induces anxiety-like behavior in mice.
To investigate whether the changes in GABACeA neuronal activity over time in ARS-2h mice are related to microglial activation, researchers examined the activation of microglia in the CeA using immunofluorescence detection of the microglia-specific marker Iba1. At 0.5 and 8 hours post-ARS-2h, the number of Iba1+ microglia in model mice was significantly higher than in corresponding control mice. Notably, these activation phenotypes returned to normal levels at 12 hours post-ARS-2h, which correlated strongly with the pattern of neuronal activity. Subsequently, they found that microglial activation in the CeA was accompanied by increased expression of MHCII and inflammatory factors such as Tnf-α, Il-1β, and Il-6. Additionally, TUNEL assays showed that apoptosis levels peaked at 8 hours post-ARS-2h and then returned to baseline levels at 12 hours(For more data, please refer to the supplementary materials of the original text). These results led us to hypothesize that microglial activation in the CeA induced by acute stress might be involved in the extinction of anxiety-like behaviors. Minocycline, a drug that inhibits microglial activity, was used, and Iba1 immunofluorescence staining confirmed that microglial activation in the CeA was significantly inhibited in mice pre-treated with minocycline before ARS-2h induction. Compared to ARS-2h mice pre-treated with saline, the spontaneous neuronal activity of GABACeA neurons at 12 hours post-ARS-2h was significantly higher. These results indicated that inhibiting microglial activation blocks the recovery of GABACeA neuronal hyperactivity and the extinction of anxiety-like behaviors induced by acute stress.
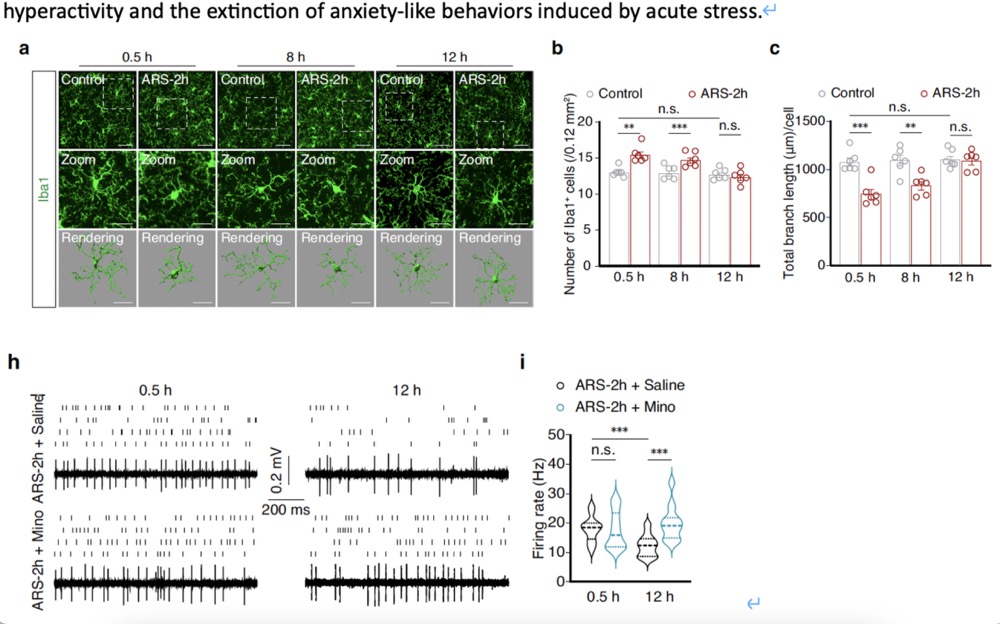
Fig2. Microglial activation mediates changes in anxiety-like behavior following stress.
The researchers examined the remodeling of GABACeA neuronal dendritic spines in model mice after ARS-2h by injecting AAV-CSSP-YFP-8E3 virus(provided by Brain Case Biotech) into the bilateral CeA of GAD2-Cre mice, specifically labeling the dendritic spines of GABACeA neurons. The dendritic spine density of GABACeA neurons in model mice increased at 0.5 hours and 8 hours post-ARS-2h but returned to control levels at 12 hours post-ARS-2h. However, in model mice pre-treated with minocycline (i.p.) compared to those treated with saline, there was no significant difference in GABACeA neuronal dendritic spine density at 0.5 hours post-ARS-2h, but the dendritic spine density was significantly higher in the minocycline pre-treatment group at 12 hours post-stress induction (Fig. 3d–f). These results indicate that the enhanced phagocytosis by microglia is necessary for the observed reduction in GABACeA neuronal dendritic spine density during the extinction of anxiety-like behaviors induced by acute restraint stress. This process depends on increased contact areas between microglia and dendrites and the colocalization of GAD65/67 immunoreactive puncta with Iba1+ microglia, leading to enhanced phagocytic activity.
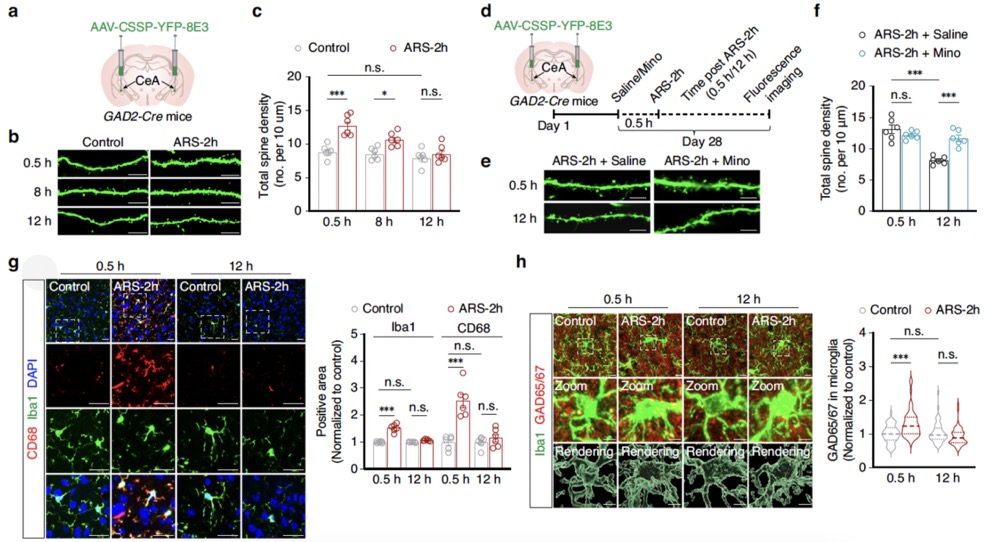
Fig3. During the disappearance of anxiety-like behavior induced by acute restraint stress, enhanced microglial phagocytosis contributes to the remodeling of neuronal dendritic spines.
Researchers found that neuronal activity significantly increased at ARS-5min, while microglial branch length and nodes did not change. However, at ARS-30min, microglial branch length and branch points significantly decreased. This result suggests that in mice subjected to acute restraint stress, the activation of GABACeA neurons precedes microglial activation. Furthermore, chemogenetic activation of GABACeA neurons in naïve mice led to microglial activation, indicated by an increased number of Iba1+ microglia and decreased branch length and points in these cells. Inhibition of GABACeA neurons abolished the observed microglial activation in ARS-2h mice. These results indicate that the activation of microglia is caused by the increased activity of GABACeA neurons induced by acute restraint stress in mice(For more data, please refer to the supplementary materials of the original article).
Previous studies have found that CX3CL1 (fractalkine) is a chemokine secreted specifically by neurons and widely involved in activity-dependent synaptic pruning. It can be cleaved into a soluble isoform that, by binding to its receptor CX3CR1, triggers microglial activation. Western blot experiments showed that the level of soluble CX3CL1 protein was significantly higher in ARS-2h mice at 0.5 hours and 8 hours post-stress compared to control mice. Concurrently, qPCR was used to detect the expression of Nf-κb mRNA, a transcription factor that regulates the expression of cytokines, including CX3CL1. When the NF-κB selective inhibitor Pyrrolidinedithiocarbamate ammonium (PDTC) was injected into the CeA of model mice, a significant reduction in Cx3cl1 mRNA expression was observed at 0.5 hours post-ARS-2h compared to control animals. These results suggest that the NF-κB signaling pathway may be involved in regulating the expression of CX3CL1 protein under acute stress conditions.
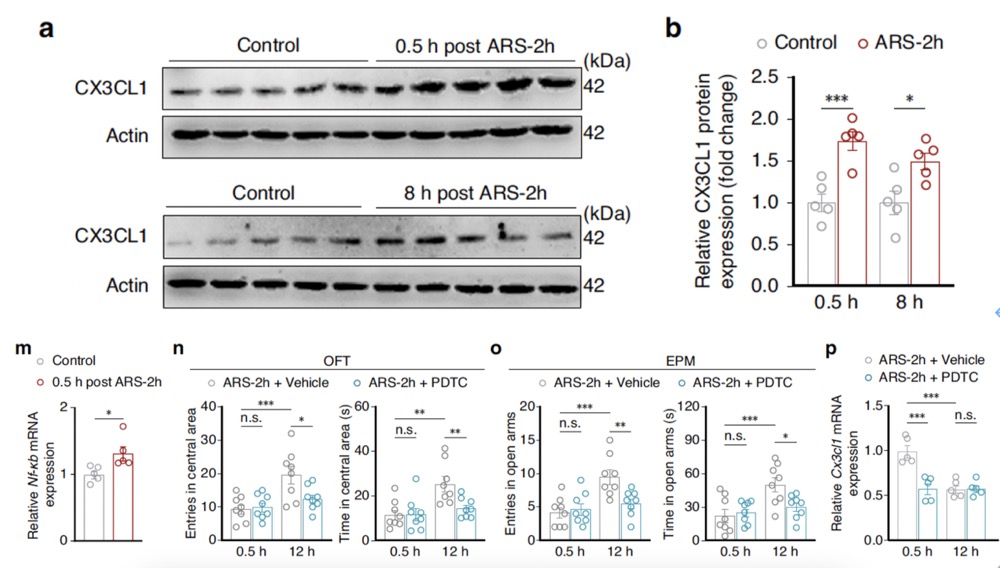
Fig4. The NF-κB signaling pathway may be involved in regulating the expression of CX3CL1 protein under acute stress conditions.
MST4 is considered a "brake" on TLR-TRAF6-NF-κB-mediated inflammatory responses. Researchers found that in the CeA, MST4 primarily colocalizes with GABA-specific antibody but not with microglia. qRT-PCR and Western blot experiments showed that compared to non-stressed control mice, MST4 expression in the CeA was significantly decreased at both mRNA and protein levels at 0.5 hours post-ARS-2h and returned to baseline levels at 12 hours post-ARS-2h. Further investigation revealed that GABACeA neuron-specific overexpression of MST4 increased the duration of stay and number of entries in the central area of the OFT and open arms of the EPM in ARS-2h mice. GABACeA neuronal activity was restored in ARS mice, accompanied by significantly reduced levels of Nf-κb and Cx3cl1 mRNA, decreased microglial activation characterized by fewer Iba1+ cells, and increased total microglial branch length and points.
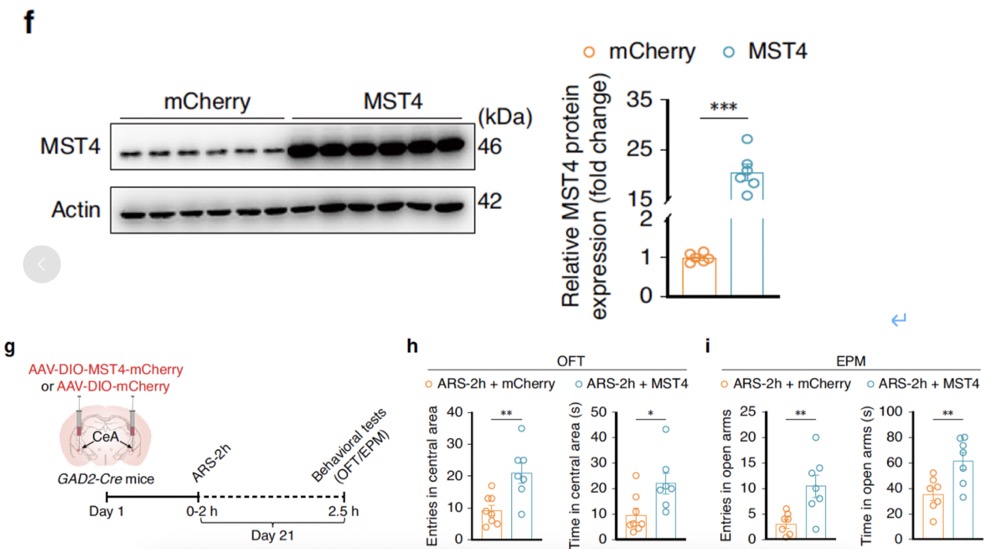
Fig5. GABACeA neuron-specific overexpression of MST4 is associated with significantly reduced levels of Nf-κb and Cx3cl1 mRNA, decreased microglial activation characterized by fewer Iba1+ cells, and increased total microglial branch length and points.
Conversely, GABACeA neuron-specific knockdown of MST4 led to significantly increased levels of Nf-κb and Cx3cl1 mRNA, along with elevated CX3CL1 protein expression in GABACeA neurons. This condition also triggered microglial activation and significantly increased engulfment of GABACeA neuronal spines. These results indicate that downregulation of MST4 expression after acute restraint stress activates NF-κB-CX3CL1 signaling in GABACeA neurons, promoting microglial activation and engulfment of GABACeA neuronal spines, thereby restoring GABACeA neuronal hyperactivity and reducing anxiety-like behaviors.
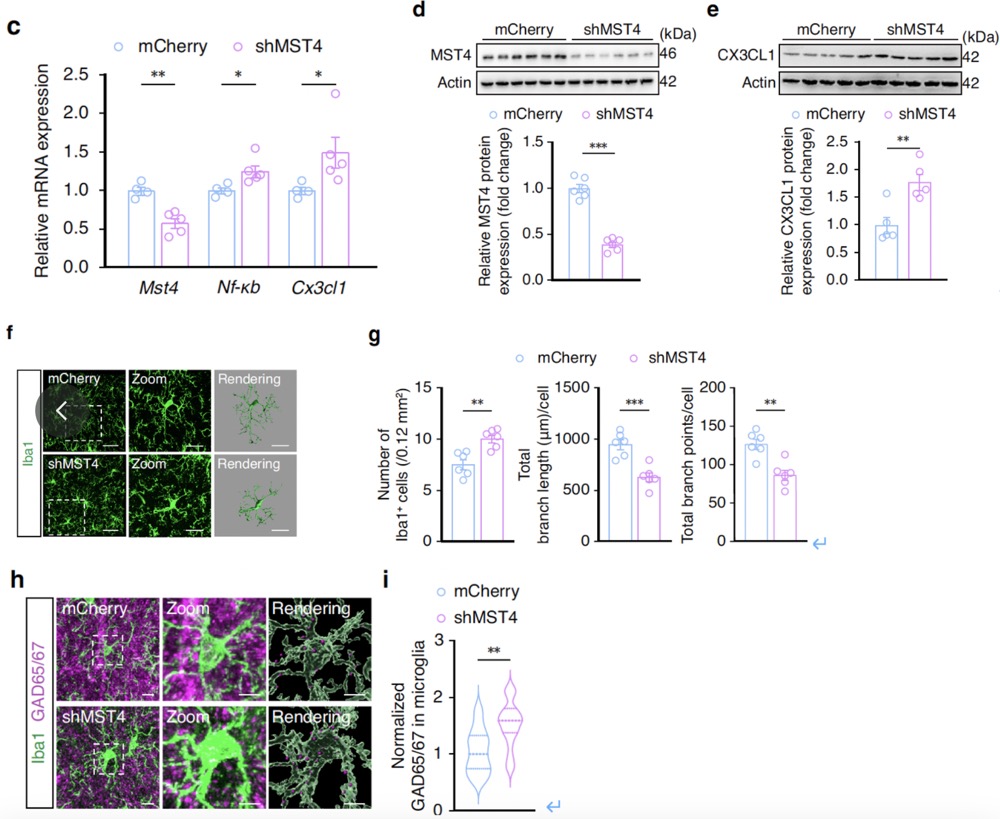
Fig6. GABACeA neuron-specific knockdown of MST4 leads to significantly elevated levels of Nf-κb and Cx3cl1 mRNA, along with increased expression of CX3CL1 protein in GABACeA neurons. This situation also triggers microglial activation and significantly increases the phagocytosis of GABACeA neuron spines by microglia.
Catalog Number. BC-SL003
Product Name. CSSP-YFP-8E3
Note: The researchers examined the remodeling of GABACeA neuronal dendritic spines in model mice after ARS-2h by injecting AAV-CSSP-YFP-8E3 virus into the bilateral CeA of GAD2-Cre mice, specifically labeling the dendritic spines of GABACeA neurons.