- E-mail:BD@ebraincase.com
- Tel:+8618971215294
On May 20, 2024, the research teams of Researcher Li Huiyan, Associate Researcher Zhang Yucheng, and Researcher Zhou Tao from the Academy of Military Medical Sciences published a research paper titled "Innervation of nociceptor neurons in the spleen promotes germinal center responses and humoral immunity" online in the journal Cell (Impact Factor: 64.5). This study is the first to reveal the complete three-dimensional structure of nociceptive nerve fibers in the spleen and identify a sensory neural circuit between the dorsal root ganglion (DRG) and the spleen. Activation of this pathway can significantly enhance humoral immunity and the host's ability to defend against viruses. These findings not only deepen the understanding of the interaction and regulation between peripheral nerves and humoral immunity but also open a new avenue for the treatment of B-cell-mediated autoimmune diseases.

The spleen, a highly vascularized organ encased in a dense fibrous connective tissue capsule, is innervated by nociceptor neurons. To determine if the spleen is under the control of these neurons, the authors employed a tissue processing method called SHANEL (Small Micelle-Mediated Human Organ Efficient Clearing and Labeling). This technique effectively clears and labels dense or blood-rich organs. Using SHANEL to clear and stain the entire spleen, the researchers detected the specific marker of nociceptor neurons, CGRP. They then used light-sheet microscopy for 3D imaging and observed that the spleen is innervated by CGRP+ nociceptor neurons, which form a complex and extensive structure within the spleen.
To further confirm this phenomenon, the authors crossed Trpv1-Cre mice with iEGFP reporter mice, labeling TRPV1+ nociceptors with enhanced green fluorescent protein (EGFP). The results showed that TRPV1-Cre+ and CGRP+ nociceptor nerve fibers innervate the spleen.
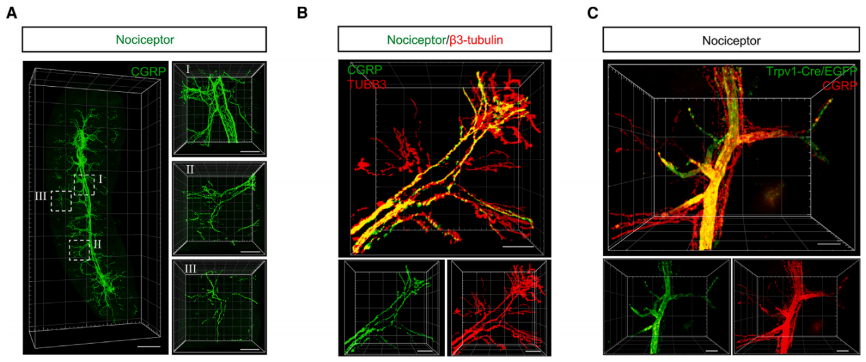
Fig1. (A) Representative 3D reconstructed light sheet images of nociceptive nerve fibers (CGRP, green) in the SHANEL-cleared whole spleen. (C) 3D reconstructions of TRPV1-EGFP+ (green) and CGRP+ (red) nociceptive nerve fibers in the advanced CUBIC-cleared spleen from the Trpv1-Cre+iEGFP+ mouse.
To map the spatial distribution of nociceptive nerves in the spleen, CGRP was co-immunolabeled with the specific vascular endothelial cell marker CD31. The results showed that CGRP+ nociceptive nerve fibers primarily travel along the vascular network of the spleen. CGRP+ nociceptive nerves enter the spleen parenchyma from the splenic hilum and then follow the vascular branches into the white pulp (WPs). Importantly, when the spleen was co-labeled with the B cell marker B220, abundant CGRP+ nociceptive nerves were observed in the B cell areas. Additionally, it was found that CGRP+ nociceptive nerve fibers are in close proximity to B cells in the spleen. Therefore, these data reveal that nociceptive nerves can penetrate deep into the spleen parenchyma, particularly along the blood vessels, and reach the B cell areas.
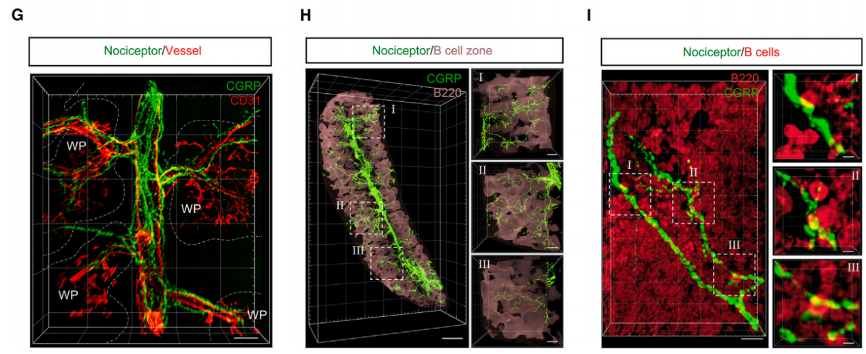
Fig.2 3D reconstructed light sheet images of nociceptive nerve fibers (CGRP, green), blood vessels (CD31, red), and B cell zones (B220, pink) in the SHANEL-cleared spleen.
To investigate the role of nociceptors in humoral immune responses, immunizations were induced using NP-KLH in DT-treated Trpv1-Cre- iDTR+ or Trpv1-Cre+ iDTR+ mice. This induction triggered GC responses. On the 13th and 28th days after NP-KLH immunization, Trpv1-Cre+ iDTR+ mice lacking nociceptors exhibited significantly reduced germinal center (GC) B cells, plasma cells, NP-specific GC B cells, and NP-specific memory B cells, along with a significant decrease in antibody titers. Additionally, it was observed that CGRP+ nociceptor nerve fibers densely surrounded and extended into the splenic germinal centers in Aicda-Cre+ itdTomato+ mice immunized with NP-KLH. These results indicate that nociceptor neurons play a crucial role in splenic GC responses and humoral immunity.
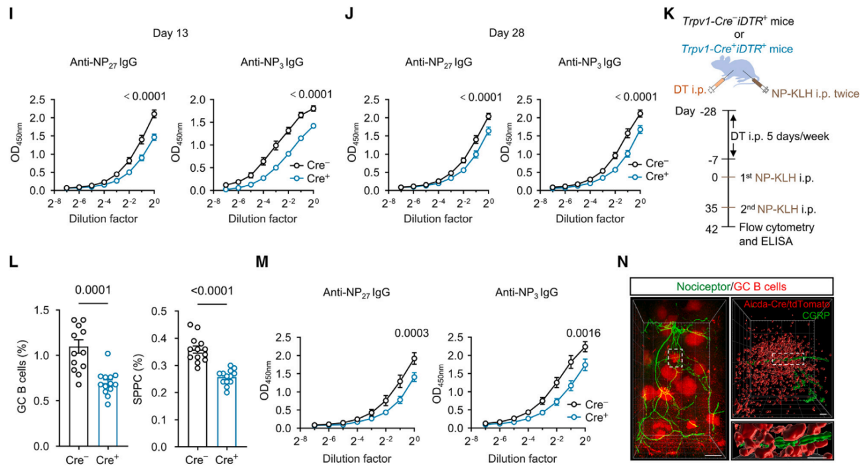
Fig3. After NP-KLH induction immunization, Trpv1-Cre+ iDTR+ mice lacking nociceptors exhibited a significant decrease in germinal center (GC) B cells, plasma cells, NP-specific GC B cells, and NP-specific memory B cells, along with a significant decrease in the titers of related antibodies. Additionally, it was found that CGRP+ nociceptive nerve fibers extended into the Aicda+ splenic GC.
To trace the origin of nociceptive nerve fibers in the spleen, pseudo-rabies virus (PRV) retrograde tracing combined with immunostaining using antibodies against TRPV1 or CGRP was employed to detect the cell bodies of nociceptive neurons located in the DRGs or NGs. The results revealed that nociceptive neurons in the spleen (PRV+ TRPV1+) are mainly distributed in the DRGs rather than the bilateral NGs. The cell bodies of TRPV1+ nociceptive neurons in the spleen are primarily located in the left thoracic (T8-T13) DRGs on the same side as the spleen, with few found in adjacent (T6-T7 and L1-L2) and contralateral (right) DRGs. The findings indicate that the majority of nociceptive nerve fibers in the spleen originate from the left T8-T13 DRGs.
To further confirm the retrograde tracing results, AAV9-Trpv1-Cre virus was injected for anterograde tracing into the left T8-T13 DRGs of itdTomato+ mice. Combining CUBIC tissue clearing technique with light sheet imaging, the results showed that TRPV1-tdTomato+ CGRP+ nociceptive nerve fibers innervate the spleen, suggesting direct innervation of the spleen by nociceptive neurons in the left T8-T13 DRGs.
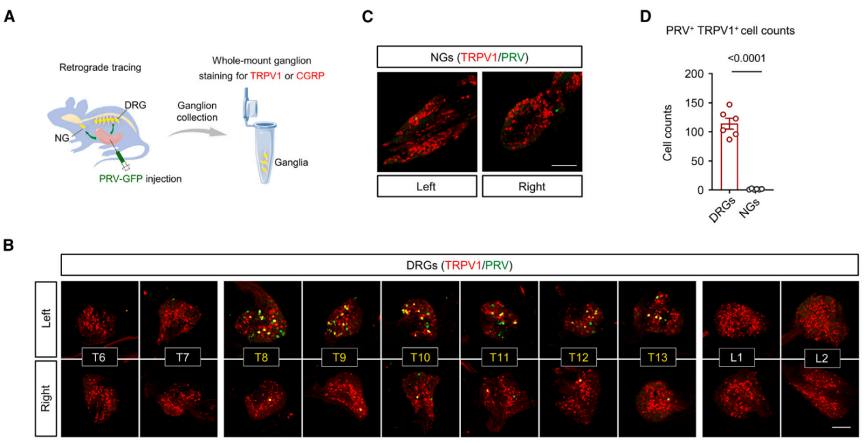
Fig4. The spleen was injected with PRV (Pseudorabies Virus. The BC-PRV-531 virus was obtained from Brain Case Biotechnology), retrogradely tracing to the entire spinal ganglia and sympathetic ganglia, where PRV-EGFP expressed green fluorescence, and anti-TRPV1 antibody displayed red fluorescence.
Data shows that defects in nociceptors in the left T8-T13 DRGs mediated by the capsaicin analog RTX result in a significant reduction in the production of GC B cells, SPPCs, and NP-specific antibodies after NP-KLH immunization in mice. However, eliminating TRPV1+ neurons in the right T8-T13 DRGs or bilateral NGs does not affect the immune response and antibody titers. Injecting AAV9 virus expressing the hM3D (Gq) receptor into the left T8-T13 DRGs of Trpv1-Cre+ mice, followed by intraperitoneal injection of CNO to activate the receptor, chemogenetically activates TRPV1+ nociceptive neurons. Phosphorylated extracellular signal-regulated kinase (p-ERK) indicates that intraperitoneal injection of CNO specifically activates TRPV1+ neurons in the left T8-T13 DRGs without affecting the ratio of splenic B cells and T cells. After NP-KLH immunization, mice administered with CNO show higher proportions of splenic GC B cells, SPPCs, and NP-specific IgG antibody levels compared to control mice, indicating that activation of nociceptors in the left T8-T13 DRGs significantly enhances the splenic GC response. In summary, nociceptive neurons from the left T8-T13 DRGs play a critical role in splenic GC response and humoral immunity.
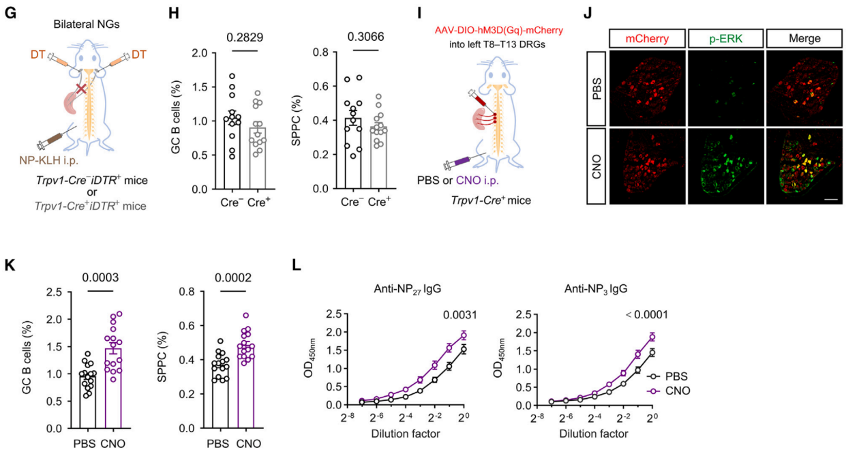
Fig5. (G) Schematic of detecting the effect of vagal nociceptor ablation on the GC response. (H) Percentages of splenic GC B cells and SPPCs on day 13 after immunization in control or vagal-nociceptor-ablated mice. n = 12 and 13 mice, respectively. (I) Schematic of chemogenetic activation strategy for nociceptors in left T8–T13 DRGs through hM3D(Gq) expression and CNO injection in Trpv1-Cre+ mice. (J) Representative immunofluorescence images of DRGs infected with AAV9-DIO-hM3D(Gq)-mCherry virus from Trpv1-Cre+ mice intraperitoneally (i.p.) injected with PBS or CNO. Red, mCherry; green, p-ERK. (K) and ELISA titration curves of NP-specific IgG antibodies. (L) in PBS- or CNO-treated mice on day 13 after NP-KLH immunization.
Nociceptors innervating visceral organs can be directly activated by resident microbial pathogens and their products. Upon activation of nociceptor nerve endings, local release of neuropeptides, such as calcitonin gene-related peptide (CGRP), occurs, exerting direct effects on target cells to regulate downstream physiological functions. Results indicate that accumulation of prostaglandin E2 (PGE2) in the spleen after antigen immunization enhances nociceptor activity, leading to induction of CGRP release within the spleen following antigen invasion. Mechanistic studies have revealed that CGRP regulates GC responses and antibody generation by acting on CALCRL/RAMP1 receptors on B cells. Since spicy foods contain natural agonists of nociceptive nerves - capsaicin, researchers found that capsaicin feeding specifically activates mouse nociceptive nerves, thereby enhancing splenic GC responses and antibody production levels, ultimately enhancing resistance to influenza virus infection.
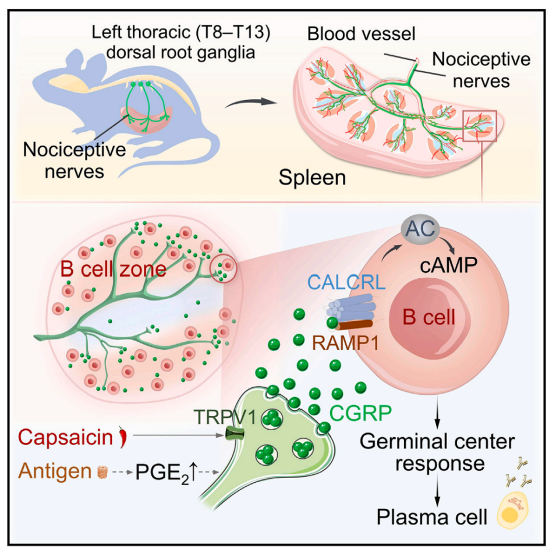
Fig6. The spinal cord nociceptors innervate the spleen along blood vessels, transmitting regulatory signals to B cells via the CGRP-CALCRL/RAMP1 axis, promoting immune responses and host defense.
In summary, this study enriches the understanding of the regulatory function of peripheral nerves, especially nociceptors, in adaptive immunity, and makes important contributions to understanding the mechanisms of neuroimmune interactions. Moreover, this study provides new therapeutic avenues for targeting nociceptive nerves, CGRP neuropeptides, and their receptors to treat autoimmune diseases characterized by aberrant B cell activation.
The virus products used in this study from Brain Case Biotech are listed below:
|
Product Type |
Product Number |
Product Name |
|---|---|---|
|
Product Name |
PRV-CAG-EGFP |
|
|
Chemogenetics |
rAAV-EF1α-DIO-hM3D(Gq)-mCherry |
|
|
Recombinase |
rAAV-TRPV1-SV40 NLS-Cre |