- E-mail:BD@ebraincase.com
- Tel:+8618971215294
The sleep–wake cycle, including rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep, is generally considered to be primarily regulated by distributed subcortical circuits, whereas cortical regions are involved in generating sleep-related electroencephalogram (EEG) oscillations. However, recent studies showed that cortical neurons could be actively involved in NREM sleep generation and homeostatic sleep regulation. Although cortical activation during REM sleep is considered to be associated with vivid dreaming, the role for the cortex in REM sleep regulation remains unknown. The transition of activity patterns temporally matched the transition of two sequential REM substages, identified by contrasting facial movement and distinguishable EEG theta oscillation. Closed-loop optogenetic inactivation of excitatory neurons in RSC during REM sleep shortened REM sleep duration, via inhibition of the REM substage transition. By contrast, inactivation of other cortical areas—the secondary motor cortex (M2) or the anterior cingulate cortex (ACC)—had no effect on REM sleep duration. These results uncover two distinct substages in REM sleep and highlight a role for RSC in regulating REM substage transition.
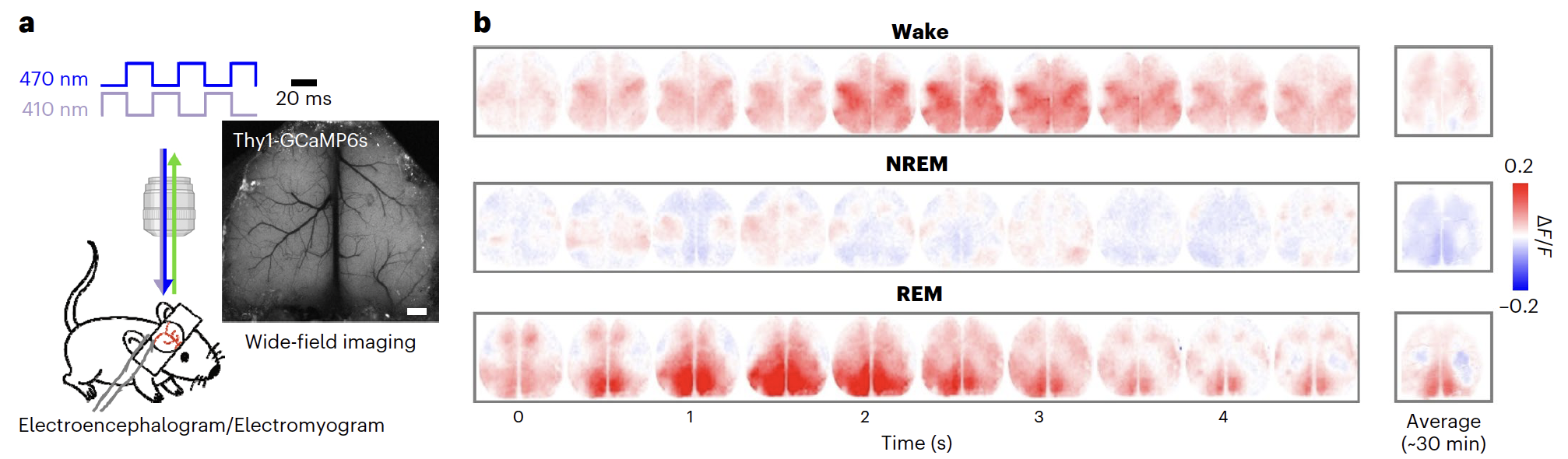
Fig 1. Researchers implanted a chronic transparent window covering the entire dorsal cortex of the Thy1-GCaMP6s mice and recorded Ca2+signals together with EEG, electromyogram (EMG) and video recording. They found that cortical activity during REM sleep was higher than that during other sleep states, with posterior and medial cortical regions more active, these results indicate that the RSC was selectively activated during REM sleep.
By inspecting mouse facial characteristics during REM sleep, we found that the eye, whisker and cheek movements also exhibited two distinguishable patterns
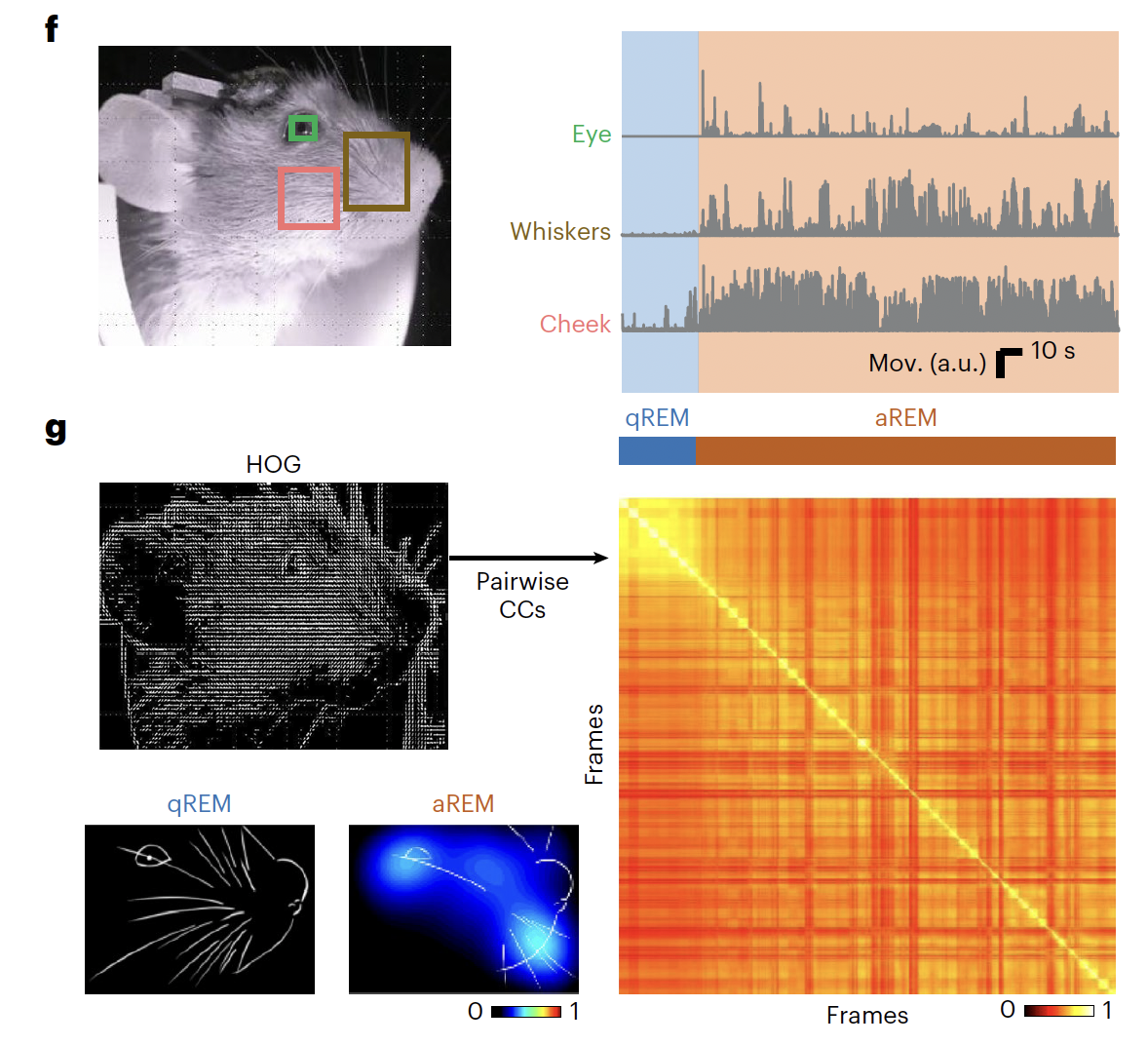
Fig2: The results revealed two main clusters during REM sleep, corresponding to a state with and a state without active facial movements, defined here as active REM (aREM) and quiescent REM (qREM) sleep, respectively.
To examine whether the RSC plays an active role in REM sleep, researchers performed optogenetic inactivation experiments. They first characterized the effect of RSC inactivation on cortical dynamics. Adeno-associated virus expressing halorhodopsin (NpHR) fused with mCherry (AAV-CaMKIIα-NpHR3.0-mCherry) was injected bilaterally into the L2/3 of the RSC in Thy1-GCaMP6s mice, allowing simultaneous GCaMP6s imaging (with blue light) and RSC inhibition.
We found that RSC activity was markedly reduced by the optogenetic inactivation during REM sleep. By analyzing cortex-wide Ca2+ activities, under RSCREM inactivation, we observed two new motifs with Ca2+ activity initiated at FrA, followed by either sustained local FrA activation or propagated waves to posterior cortical regions. These data showed that RSC inactivation markedly reorganized global cortical activity dynamics during REM sleep.
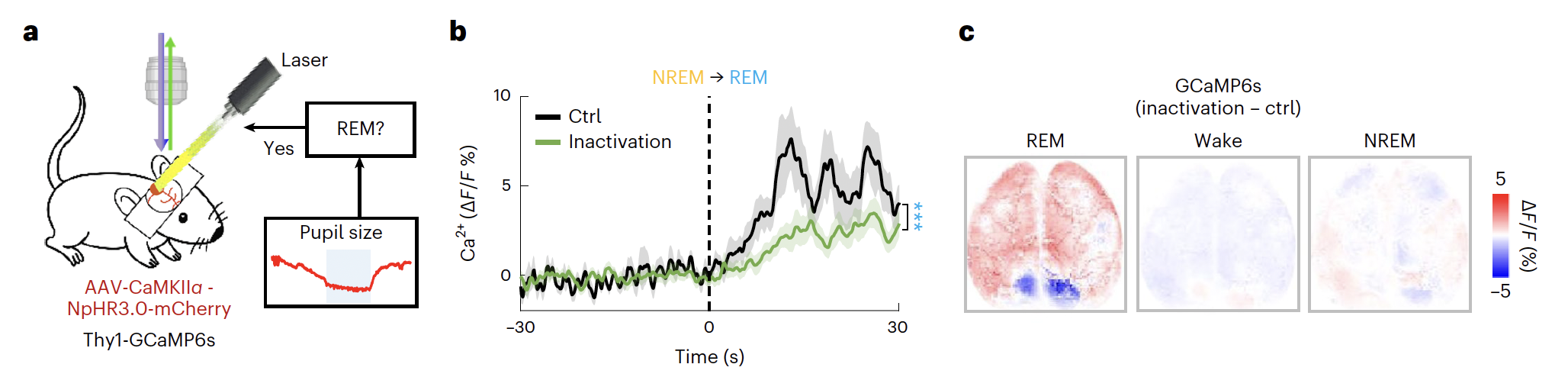
Fig 3. Adeno-associated virus expressing halorhodopsin (NpHR) fused with mCherry (AAV-CaMKIIα-NpHR3.0-mCherry) was injected bilaterally into the L2/3 of the RSC in Thy1-GCaMP6s mice, allowing simultaneous GCaMP6s imaging (with blue light) and RSC inhibition.
To achieve high inhibition efficiency for behavioral measurements, we next injected RSC L2/3 bilaterally with AAV expressing light-gated chlorine channel GtACR2 (AAV-CaMKIIα-GtACR2-EGFP). They found that RSCREM inactivation markedly reduced the duration of aREM sleep and increased the duration of qREM sleep, resulting a shortening of total REM duration. This effect of RSCREM inactivation was largely due to the inhibition of qREM-to-aREM transitions, rather than simply the induction of wakefulness, indicating that aREM initiation is crucial for maintaining REM sleep. RSC neurons were also important for aREM sleep maintenance, as RSCaREM inactivation largely reduced aREM duration.
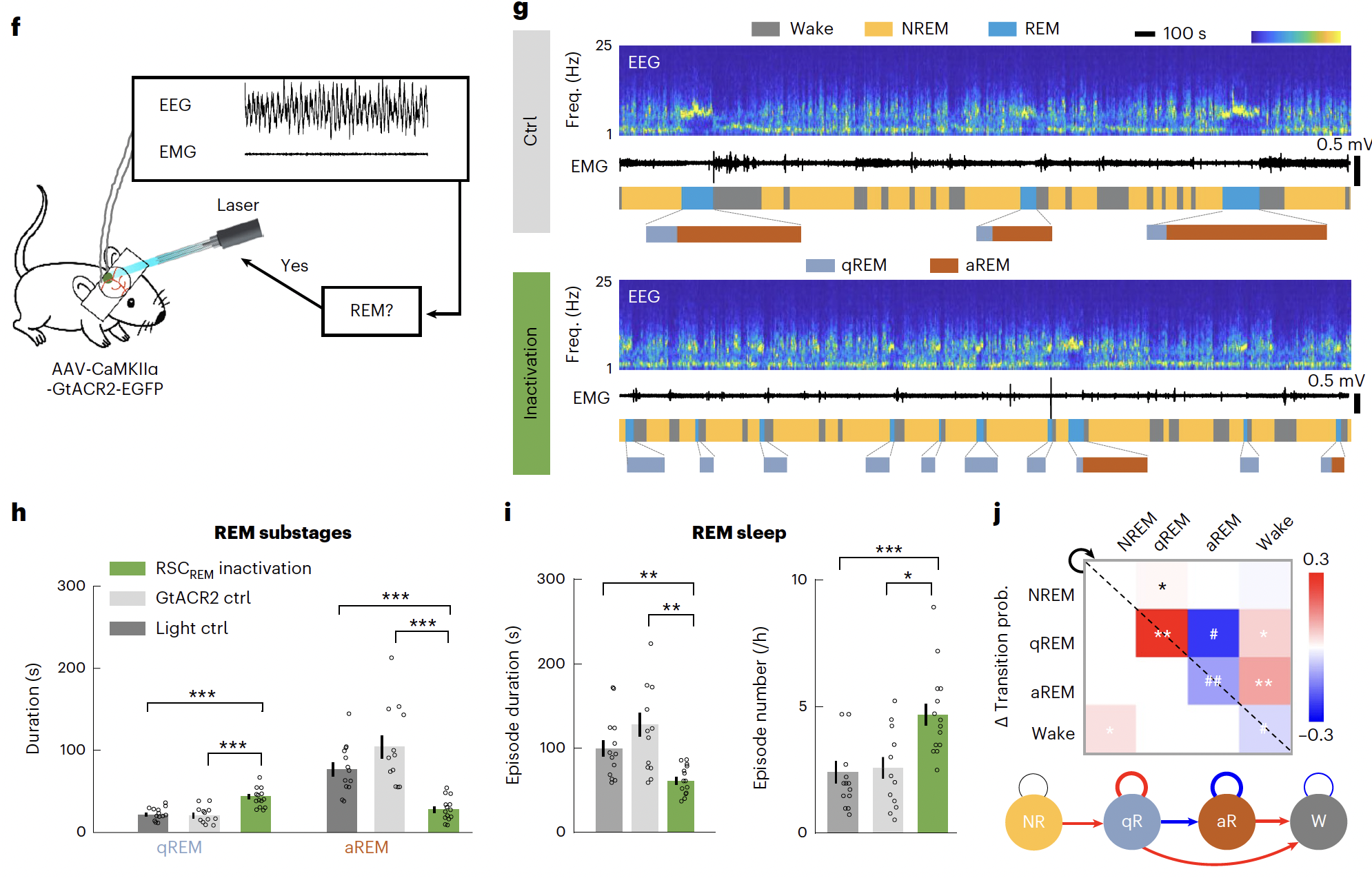
Fig.4 RSCREM inactivation markedly reduced the duration of aREM sleep and increased the duration of qREM sleep, resulting a shortening of total REM duration. This effect of RSCREM inactivation was largely due to the inhibition of qREM-to-aREM transitions, rather than simply the induction of wakefulness, indicating that aREM initiation is crucial for maintaining REM sleep
Article Source:
https://www.nature.com/articles/s41593-022-01195-2
AAV9-hSyn-DIO-GCaMP6s
AAV9-CaMKIIα-NPHR3.0-mCherry
AAV9-hSyn-DIO-GtACR2-EGFP
AAV9-CaMKIIα-GtACR2-EGFP
AAV9-CaMKIIα-mCherry
AAV9-CaMKIIα-EYFP
AAV9-hSyn-jRGECO1a