- E-mail:BD@ebraincase.com
- Tel:+8618971215294
The global lifetime prevalence of PTSD is 1.9-6.8%, and traditional exposure therapy or drug treatment has no significant effect on 40% of patients. The disease affects a large number of patients and requires long-term training and treatment as well as continuous social support, placing a huge economic and ethical burden on society and patients' families. Clinically, PTSD patients often show excessive defensive responses to relatively small stimuli and show irritated behaviors. How to effectively improve the excessive defensive behavior of PTSD patients is an urgent problem around the world.
On February 3, 2023, the Wu Shengxi/Guo Baolin team from the School of Basic Medicine of Air Force Medical University published research results titled "Reversal of hyperactive higher-order thalamus attenuates defensiveness in a mouse model of PTSD" in the journal Science Advances. The research team used the SPS&S mouse model to construct a PTSD model mouse that can effectively fit the excessive defensive behavior of PTSD patients. Through various adjustment methods such as optogenetics and chemical genetics, the loop regulates neurons in the higher-order thalamus. activity, effectively improving the excessively enhanced defensive behavior of PTSD model mice.

The researchers found that PTSD model mice had excessive defensive behaviors induced by whisker stimulation and trauma-related environments. During the defensive behavior, the neuronal activity of the higher-order thalamus-posteromedial nucleus of the thalamus (PoM) in PTSD model mice was significant. At the same time, the activity of neurons in the thalamic reticular nucleus (TRN), the source of its inhibitory upstream input, is reduced. The anterior association cortex (FrA) is a brain area related to defense downstream of PoM.
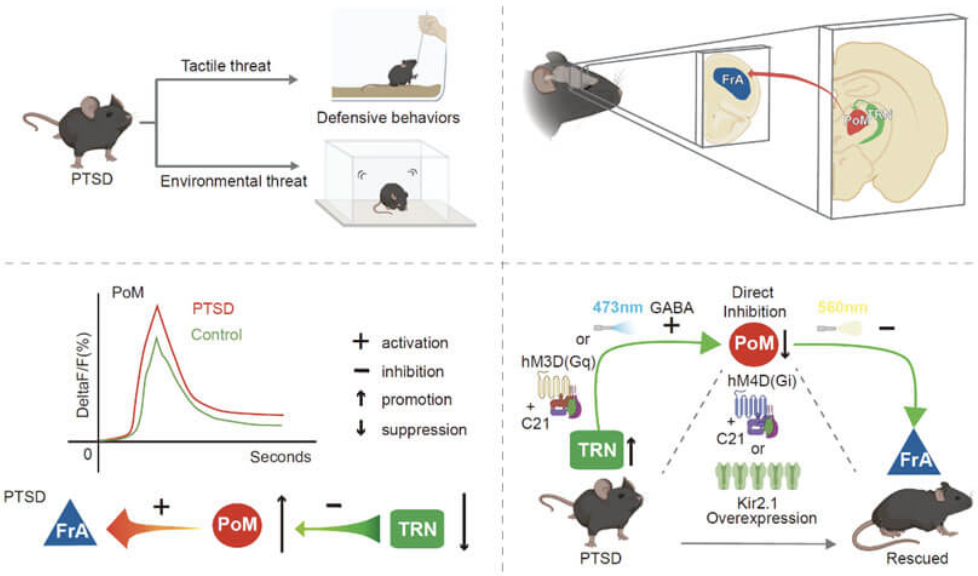
By overexpressing the inward rectifier potassium channel Kir2.1 in the PoM in situ or chemically genetically inhibiting the neuronal activity of the PoM, the excessively enhanced defensive behavior of PTSD model mice can be effectively improved. At the same time, specifically activating TRN neurons that project to the PoM through chemical genetics or optogenetically activating the axon terminals of TRN that projects to the PoM brain area can effectively improve the excessively enhanced defensive behavior of PTSD model mice.
Moreover, this study used rabies virus retrograde tracing technology and Fos TRAP technology to verify for the first time that the anterior association cortex FrA is a brain area downstream of PoM related to defensive behavior, and specifically inhibited the axon terminals of PoM projecting to FrA through optogenetics. , can effectively improve the excessively enhanced defensive behavior of PTSD model mice.
Accordingly, the research team's experiments on PTSD model SPS&S mice confirmed that circuit regulation of higher-order thalamus can effectively improve the excessive defensive behavior of PTSD, providing a theoretical basis for potential clinical targeted treatments.
Original link: https://www.science.org/doi/10.1126/sciadv.ade5987
The list of Brain Case Biotech virus products used in this article is as follows: