- E-mail:BD@ebraincase.com
- Tel:+8618971215294
A deep understanding of the neurological principles behind different behaviors helps us understand the biological basis of the brain. Some seemingly simple behaviors often have complex neural regulation mechanisms behind them. Feeding is a top priority for survival. During the course of hundreds of millions of years of evolution, species have been living in complex and ever-changing natural environments. On the one hand, they need to be keenly aware of food clues, and on the other hand, they also need to be constantly vigilant and pay attention to changes in the surrounding environment. Natural selection gradually shaped sophisticated feeding strategies and solidified them into the brain's neural networks. However, due to the lack of sophisticated behavioral analysis methods, researchers have long mainly used the index of food intake to evaluate feeding behavior. It has been found that a variety of neurons in dozens of brain areas are involved in the regulation of food intake [2-5]. However, to accurately describe the entire process of eating behavior, how multiple types of neurons in various brain areas work together , so far no reports.
On March 15th, Beijing time, Wang Liping’s team at the Institute of Brain Cognition and Brain Diseases, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, published a research paper titled “An iterative neural processing sequence orchestrates feeding” online in Neuron magazine [1], which was detailed for the first time. Describes the characteristics of fragmented feeding behaviors that occur cyclically between feeding and non-feeding behaviors in mice, and elucidates that three groups of neurons located in the hypothalamus and brainstem sequentially regulate the "preparation-initiation-maintenance" behavioral sequence of each feeding the process of. This research is expected to provide new ideas for the study of anorexia, obesity and other diseases, and provide new research methods and theoretical frameworks for a deep understanding of the fine neural regulation mechanisms in various instinctive behaviors.
Wang Liping's team used a behavioral tracking and recording system assisted by deep learning algorithms to conduct detailed research on the spontaneous behavior of mice during the food intake phase. A deep learning algorithm was used to identify the movements of mice in single-frame videos, and a total of 14 characteristic movements were identified. These movements were divided into 8 meaningful behaviors through a clustering algorithm, and then these behaviors were divided into feeding, walking and exploring the environment. Three categories are included, and the spontaneous behavior of mice during the food intake stage is described as a cycle of a series of behaviors such as "approaching food, ingesting, leaving food, and exploring the environment" (Figure 1).
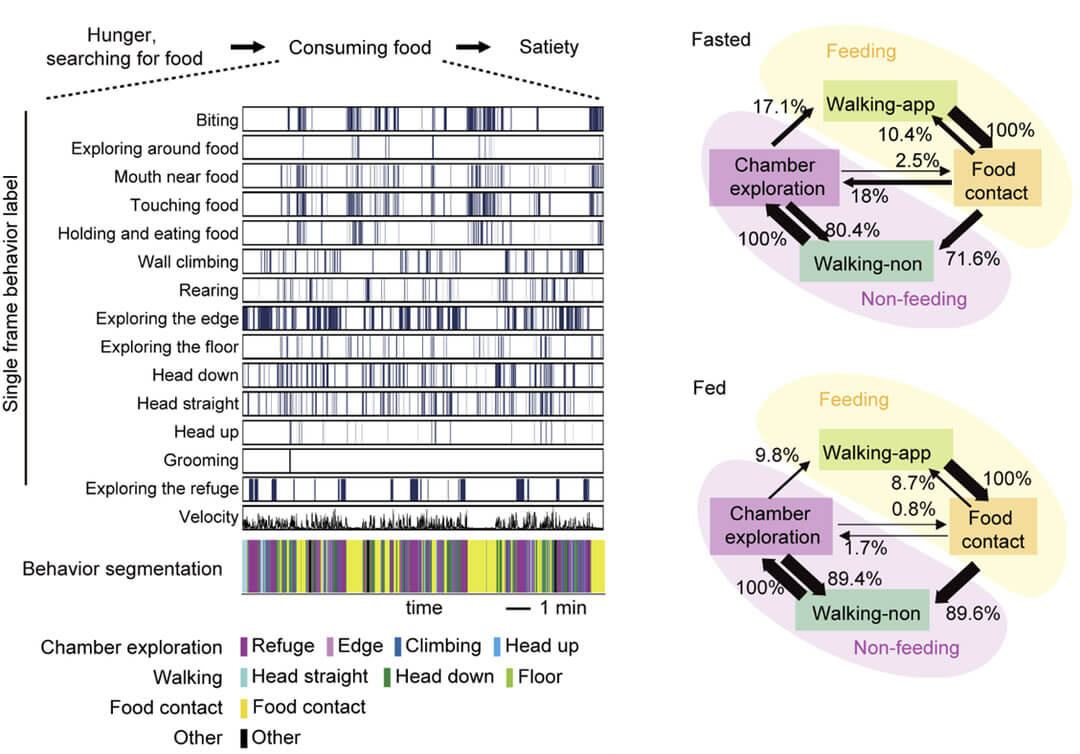
Figure 1. Using a deep learning-assisted behavior analysis system to analyze the fragmented feeding behavior of mice
By analyzing neuronal calcium responses during different spontaneous behaviors, the researchers found that ARCAgRP neurons were activated when mice were hungry and there was food in the environment, but the mice were exploring the environment without eating. It is inhibited during the feeding process; LHGABA neurons are activated when the mouse initiates feeding behavior, and the activation time has nothing to do with the duration of the feeding behavior; while DRGABA neurons are continuously activated during the feeding process, and the activation time is strongly positively correlated with the feeding time. relationship, while these neurons were inhibited when mice explored the environment away from food.
Further, the researchers used optogenetic methods to verify the functions of ARCAgRP, LHGABA and DRGABA neurons in the fragmented feeding behavior of mice. Inhibiting ARCAgRP neurons causes hungry mice to exhibit more environmental exploration and less feeding, whereas activating these neurons reduces exploration and increases feeding in the presence of food, but not in plastic sham food. The presence does not affect exploration of the environment.
Previous research has shown that ARCAgRP neurons encode negative values. It is speculated that the function of ARCAgRP neurons is to restrict ongoing behaviors unrelated to food intake under hunger conditions, thereby allowing food intake-related motivations to dominate and help initiate food intake behaviors. Activating LHGABA neurons causes mice to exhibit intense biting behavior, while inhibiting these neurons causes hungry mice to be unable to chew food. It is speculated that LHGABA neurons mediate the initiation of feeding behavior. Activating DRGABA neurons will significantly prolong the feeding behavior of mice, while inhibiting these neurons will significantly shorten the feeding behavior. It is speculated that DRGABA neurons are involved in regulating the maintenance of feeding behavior.
Therefore, ARCAgRP, LHGABA, and DRGABA neurons function sequentially to regulate the preparation, initiation, and maintenance of fragmented feeding behaviors, respectively (Fig. 2). On the one hand, this ensures the efficiency of the animal's food intake, and on the other hand, it keeps the animal alert to the environment.
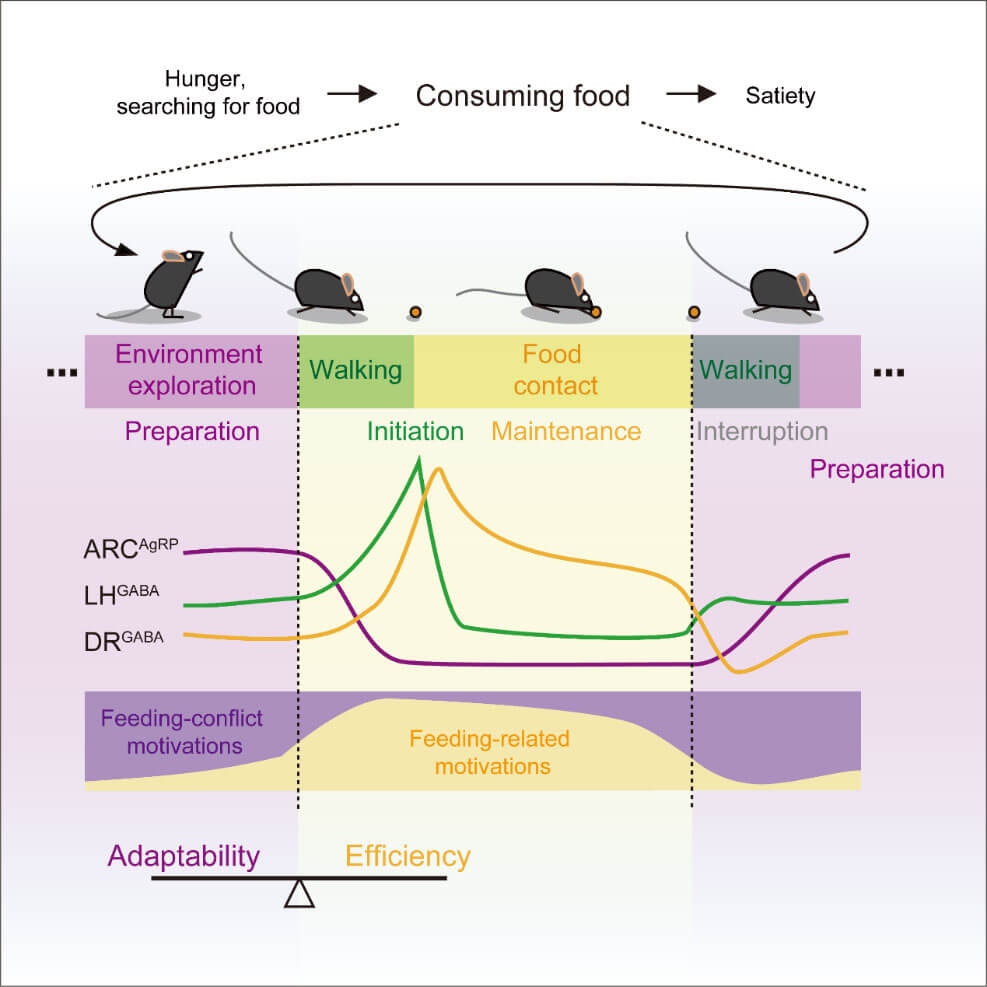
Figure 2. The preparation, initiation and maintenance of feeding episodes are regulated by ARCAgRP, LHGABA and DRGABA neurons respectively.
Similar to mice, humans also have the phenomenon of fragmented food intake. During the food intake process, they do not always pay attention to food, but constantly pay attention to the surrounding environment. Concentrating time on eating is the result of socialization training. Children mostly show "eating and playing" in the process of learning to eat independently, while adults usually engage in social activities while eating. This study deepens people's understanding of feeding behavior and the neural regulatory mechanisms during feeding, and will provide new ideas for the research and intervention of eating disorder-related diseases.
The fine behavioral analysis method established in this study is also applicable to the study of various other instinctive behaviors. Various instinctive behaviors of animals include multiple motivations competing with each other, and processes such as behavioral initiation, maintenance, and interruption by interference from other motivations. This process also involves the division of labor and cooperation of multiple groups of neurons. The external environment and the internal state of animals will dynamically regulate the response patterns of each group of neurons, thereby regulating animal behavior, allowing animals to adapt to the environment, survive and reproduce. This study lays the foundation for analyzing the fine neural regulation mechanisms at each stage of various instinctive behaviors, provides a theoretical framework for in-depth understanding of the neurocomputing mechanisms of instinctive behavioral strategies formed by animals in natural selection, and will provide a better foundation for the development of general artificial intelligence. Many theoretical basis.
Researcher Wang Liping of the Shenzhen Institute of Advanced Technology is the corresponding author of the paper. Assistant researcher Liu Qingqing, senior engineer Yang Xing and Luo Moxuan, a doctoral student jointly trained by the Institute of Advanced Technology and City University of Hong Kong, are the co-first authors of the paper. Associate Professor Rosa Chan of City University of Hong Kong and others also participated in this work. Shenzhen Advanced Institute is the first unit. The paper also received valuable opinions from Professor Fu Yu and others, and received funding from the National Natural Science Foundation of China, the Guangdong Provincial Key Areas R&D Plan and other projects.
Original link: https://www.cell.com/neuron/fulltext/S0896-6273(23)00129-0#
References
1. Liu et al. An iterative neural processing sequence orchestrates feeding. Neuron 2023 doi.org/10.1016/j.neuron.2023.02.025
2. Alcantara, IC, Tapia, APM, Aponte, Y., and Krashes, MJ (2022). Acts of appetite: neural circuits governing the appetitive, consummatory, and terminating phases of feeding. Nat. Metab. 4, 836–847 .10.1038/s42255-022-00611-y.
3. Aponte, Y., Atasoy, D., and Sternson, SM (2011). AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 14, 351–355. 10.1038/nn.2739.
4. Jennings, JH, Ung, RL, Resendez, SL, Stamatakis, AM, Taylor, JG, Huang, J., Veleta, K., Kantak, PA, Aita, M., Shilling-Scrivo, K., et al. . (2015). Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell 160, 516–527. 10.1016/j.cell.2014.12.026.
5. Nectow, AR, Schneeberger, M., Zhang, H., Field, BC, Renier, N., Azevedo, E., Patel, B., Liang, Y., Mitra, S., Tessier-Lavigne, M ., et al. (2017). Identification of a Brainstem Circuit Controlling Feeding. Cell 170, 429-442.e11. 10.1016/j.cell.2017.06.045.