- E-mail:BD@ebraincase.com
- Tel:+8618971215294
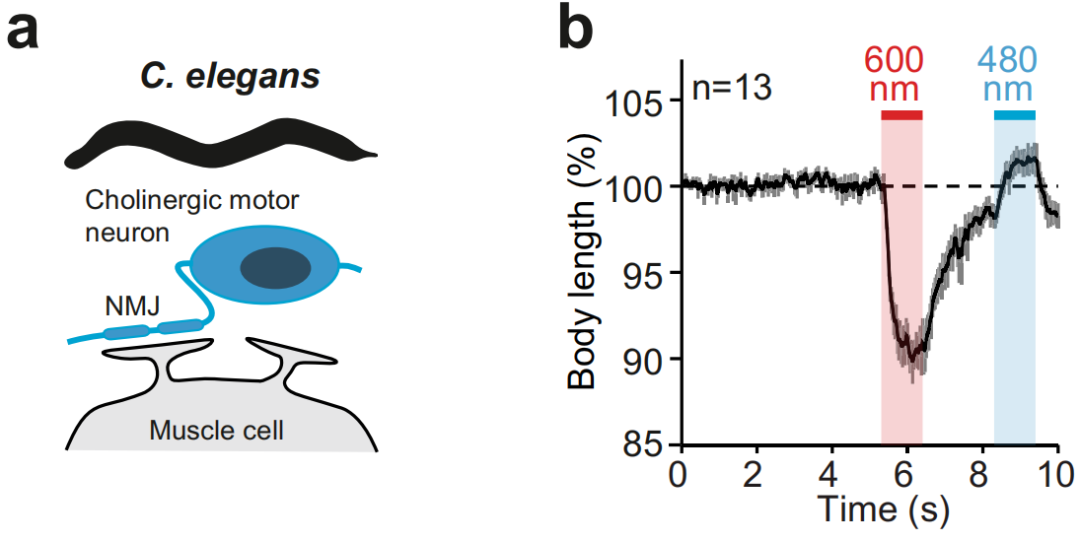
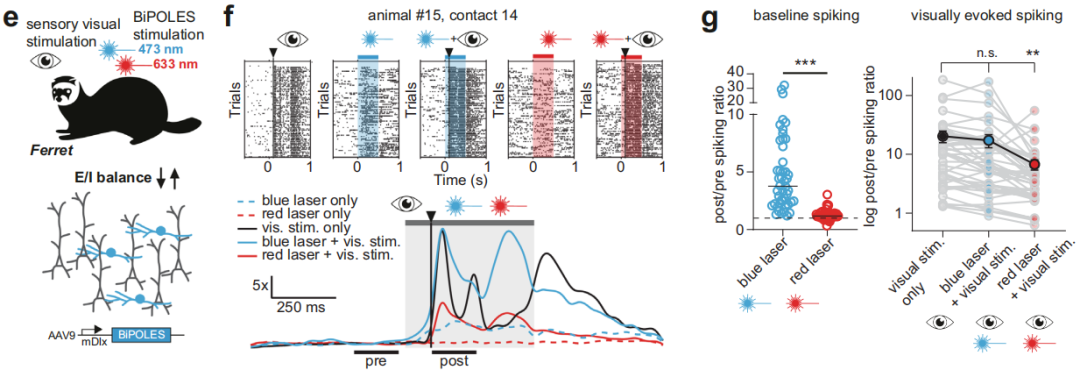
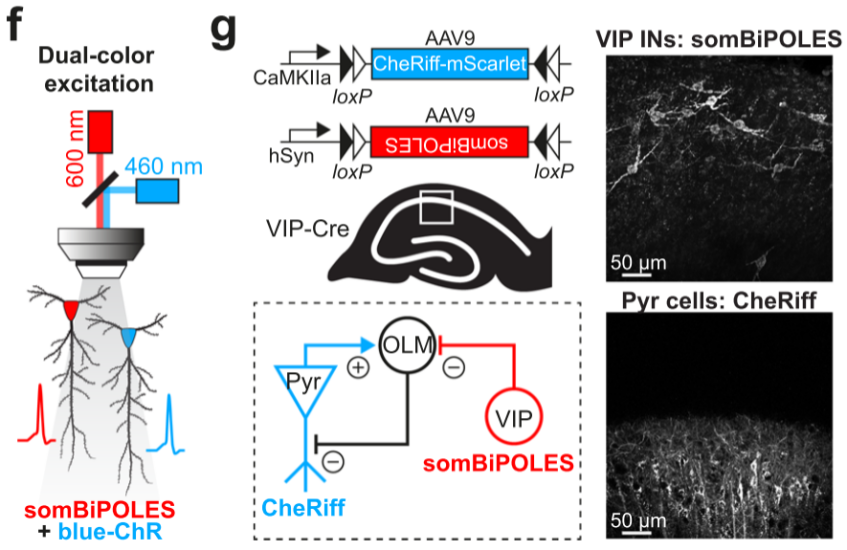
① Simultaneous regulation of different types of neurons at the same location.
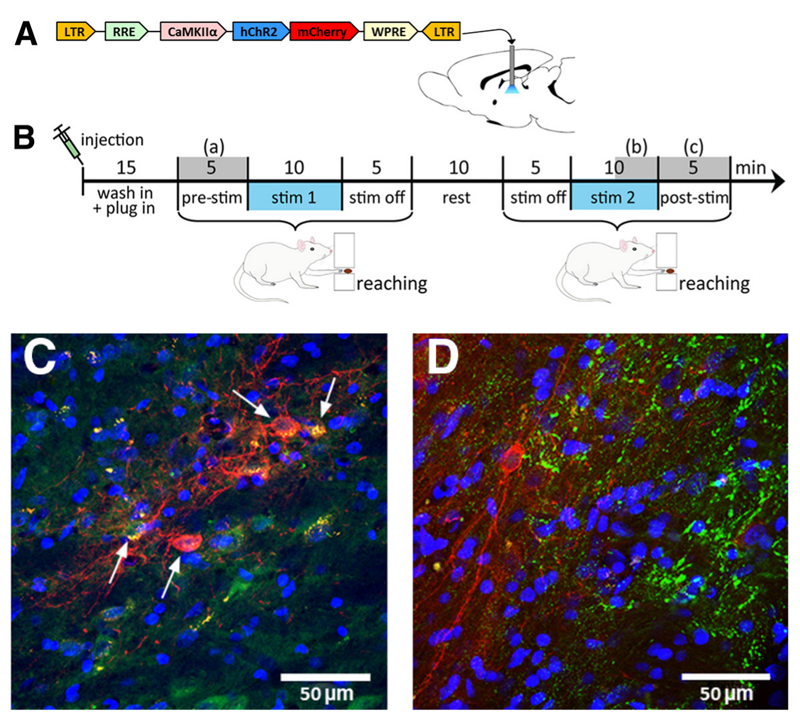
1. Optogenetic Electrophysiology: Suitable for in vivo validation of neural circuit functions.
2. Optogenetic Patch-Clamp: Suitable for ex vivo validation of neural circuit functions to determine whether functional connections exist between brain regions
3. Optogenetic Behavioral Studies: Suitable for animal behavioral research, including feeding behavior, reward behavior, anxiety and depression-related behaviors, and pain behavior.
Commonly Used Individually Expressed Photosensitive Proteins
Brain Case's current list of bidirectional optogenetic regulation products:
| Product Code | Product Name |
| BC-0948 | rAAV-nEF1α-DIO-eNPAC2.0 |
| BC-0947 | rAAV-hSyn-DIO-somBiPOLES-mCerulean3 |
| BC-1778 | rAAV-hSyn-FDIO-somBiPOLES-mCerulean3 |
| Category | Name | Description | Excitation Wavelength |
|
Optogenetics Activation Element |
hChR2(H134R) | The most commonly used ChR2, where "h" refers to the gene's coding sequence optimized with the human codon table. H134R indicates the mutation of histidine at position 134 to arginine. This mutant of ChR2 can generate twice the photocurrent, but the channel switching speed is also half as fast as the wild-type ChR2. The response frequency is ≤ 40Hz for light stimulation | 470 nm |
| hChR2 (C128S/D156A) | Also known as SSFO (Stabilized Step Function Opsins), it is an upgraded version of SFO. By introducing C128S and D156A mutations into the ChR2 gene, the channel's closing speed is significantly reduced, and it takes half an hour to close after being activated by blue light. Like SFO, the activated channel accelerates its closure when exposed to yellow light (590nm laser). Due to its slow closure, SSFO experiences cumulative effects from prolonged light exposure. Compared to hChR2, SSFO requires much lower light intensity, which reduces the thermal effects associated with prolonged light exposure and minimizes cell damage. Additionally, it increases the effective radius of blue light in tissues. SSFO is more sensitive to light than other opsin variants, allowing it to non-invasively activate neurons expressing SSFO. | 473 nm |
|
| oChIEF | oChIEF is a codon-optimized version of ChIEF. Researchers developed a channel opsin variant named ChEF by constructing a chimeric protein of ChR1 and ChR2 transmembrane domains and using site-directed mutagenesis techniques. Under continuous light stimulation, the inactivation of ChEF is significantly reduced. Compared to ChR2, which has a 77% inactivation rate, ChEF only experiences 33% inactivation. ChIEF is a version of ChEF with the mutation of isoleucine (Ile) at position 170 to valine (Val). This mutation maintains a low inactivation rate while accelerating the channel closing rate, allowing for more stable and consistent responses at stimulation frequencies above 25 Hz in HEK293 cells and cultured hippocampal neurons. Additionally, these variants also exhibit changes in spectral response, light sensitivity, and channel selectivity. Both ChEF and ChIEF enable more precise temporal control of depolarization and can induce action potential sequences that closely resemble natural firing patterns. In summary, oChIEF can respond to high-frequency light (around 100Hz) stimuli, accelerate channel closing, and reduce inactivation under continuous light stimulation in certain neurons. | 470 nm |
|
| ChETA | ChR2 E123T/H134R mutation: The E123T point mutation accelerates the closure speed of the ion channel after light removal, making the channel more sensitive to changes in light signals. The hChR2 (H134R) point mutation increases the inward current. ChETA can significantly reduce extra firing and eliminate plateau potentials, exhibiting faster kinetics. Some neurons can fire spikes at 200 Hz under laser stimulation. | 490 nm |
|
| cOpn5 | Chicken opsin 5 (cOpn5) is a super-sensitive, plug-and-play single-component photoreceptor that can control the Gq signaling pathway of cells with extremely high temporal and spatial resolution. It induces the release of stored intracellular calcium ions, thereby activating the cell. cOpn5 can effectively optogenetically activate astrocytes. | 470 nm |
|
| ChroME2s | It has a fast response speed and can activate neurons at high frequencies. The activation strength (inward current) is also larger than that of the commonly used ChrimsonR. | 1030 nm (Two-photon) |
|
| CheRiff | The maximum photocurrent displayed by CheRiff is twice that of ChR2 (H134R) or ChIEF; the opening rate is twice as fast as ChR2 (H134R) and four times faster than ChIEF; the closing rate is similar to ChIEF and 1.5 times faster than ChR2 (H134R); it enhances sensitivity and fast opening kinetics under low light intensity. These characteristics enable subcellular and low-intensity triggering of precisely timed action potentials. | 460 nm (Two-photon) |
|
|
Red-Shift Optogenetics Activation Element |
ChrimsonR | The K176R point mutation in the naturally occurring CnChR1 (Chrimson) increases the channel's closing speed, making it suitable for applications with higher stimulation frequencies. It is a new red light-driven photosensitive channel protein, with a spectral shift of approximately 45 nm redward compared to previous photosensitive channel proteins. | 590 nm |
| ChRmine | A recently discovered cation-mediated photosensitive channel protein exhibits a large photocurrent and can effectively activate neurons. Its spectral redshift allows it to be effectively activated even in deeper tissues. Additionally, it has extremely high light sensitivity, enabling it to function at lower light intensities, reducing light-induced damage to surrounding tissues. | 585 nm |
|
| hsChRmine | The H33R mutant of ChRmine exhibits enhanced fast kinetics and can fire spikes at 200 Hz under laser stimulation. | 585 nm | |
| frChRmine | The H33R/146M/G174S mutant of ChRmine exhibits enhanced fast kinetics and redshift. | 585 nm | |
| rsChRmine | The I146M/G174S double mutant of ChRmine can be activated by near-infrared light, with a significantly enhanced redshift. | 635-750 nm |
| Category | Name | Description | Excitation Wavelength |
|
Optogenetic inhibitory element
|
eNpHR3.0 | eNpHR3.0, based on NpHR, adds an endoplasmic reticulum forward transport signal and a Golgi-to-cell membrane transport signal, improving its expression on the cell membrane. Additionally, eNpHR3.0 has a more sustained current, short response time, and high sensitivity, making it one of the most widely used inhibitory photosensitive proteins. However, it has low efficiency when applied to presynaptic terminals. | 589 nm |
| eOPN3 | The targeted enhanced mosquito-derived homologous protein of vertebrate brain opsin [eOPN3, enhanced OPN3-ts-mScarlet-ER (eOPN3)] can reduce the release of neurotransmitters at the presynaptic terminal through the Gi/o signaling pathway, effectively inhibiting synaptic transmission. In both in vitro and in vivo settings, brief light exposure to the presynaptic terminal expressing eOPN3 can inhibit synaptic transmission and automatically recover within a few minutes. | 512 nm |
|
| stGtACR2 | Cell body localization enhances the membrane targeting of GtACRs (Guillardia theta anion-conducting channelrhodopsins) and reduces axonal excitation. With high photosensitivity, it significantly improves photocurrents, allowing efficient inhibition of neuronal activity in the mammalian brain. | 460 nm |
|
| ArchT | The light-driven outward proton pump, belonging to Archaerhodopsin, has a photocurrent with a similar maximum amplitude to Arch, but its light sensitivity is increased threefold. ArchT is well expressed on the membrane of cortical neurons in mice or rhesus monkeys, including long-distance transport along the neuronal axon. | 566 nm |
|
| SwiChRca | The C1C2 chimeric mutant C128A (SwiChRCA, Step-Waveform Inhibitory ChannelRhodopsin) is more sensitive to blue light and has a slow channel closing rate. As a result, short-duration light stimulation can continuously activate the channel. Red light at 632 nm can accelerate the channel's closure, terminating the neuronal inhibition state. | 475 nm |
|
| Opto-VTrap | A light-induced, reversible inhibition system that temporarily captures vesicles containing neurotransmitters from exocytosis. It can be used for the reversible inhibition of light-induced vesicle release, synaptic transmission, glial transmission, and behavior. The advantage is that it rapidly recovers within 30 minutes after the light source is turned off. Opto-VTrap can be widely applied in various cells, brain slices, and in vivo studies, and activating Opto-VTrap does not affect the membrane potential. | 488 nm |
|
| hGtACR1 | Anion Channel Rhodopsins (ACRs) are a family of light-controlled anion channels derived from green algae. They enable high-sensitivity and efficient hyperpolarization of the membrane and can mediate neuronal silencing through their light-controlled chloride conductance. | 515 nm |
|
| eArch3.0 | Arch, a light-driven outward proton pump, belongs to the Archaerhodopsin family. In neurons, it can easily achieve a suppression current of 900 pA, thereby inhibiting almost all electrophysiological activities. Although H+ ions are not the ion type used in intrinsic neural electrophysiological activities, the outward flow of H+ ions can be compensated by the cell's own mechanisms, preventing any imbalance in the intracellular and extracellular pH. Arch is generally activated by yellow-green light around 570 nm. eArch3.0 is an enhanced version of Arch that incorporates an endoplasmic reticulum forward transport signal and a Golgi-to-cell membrane transport signal, improving its expression on the cell membrane. | 590 nm |
|
| NpHR3.3 | eNpHR3.0 differs from NpHR3.3 at a single nucleotide site in its sequence. eNpHR3.0 is generally more commonly used than NpHR3.3. | 589 nm |
|
|
Redshift optogenetic inhibitory element |
Jaws | The red-shifted chloride pump belongs to the halorhodopsin family. Under 632 nm red light irradiation, it induces chloride ion influx, thereby inhibiting neuronal activity. The advantage of activating with red light is its stronger penetration capability in tissues, allowing manipulation of larger tissue areas. It can even provide light inhibition to neurons expressing Jaws from outside the skull, reducing damage to brain tissue. | 632nm |