- E-mail:bd@braincase.cn
- Tel:+86 18971216876
The production of lentivirus was mainly based on the co-transfection of three plasmids (core plasmid pLV-GOI/shRNA, helper plasmids psPAX2 and pMD2G) into HEK293T cells. The core plasmid contains the basic components of the virus, 5 'LTR and 3' LTR, as well as foreign target genes. The psPAX2 plasmid mainly carries gag, pol and rev genes, which encode the main structural proteins of the virus, specific enzymes and regulatory factors regulating the expression of gag and pol genes respectively. pMD2G vector contains vsvg gene, which provides the envelope protein needed for virus packaging.
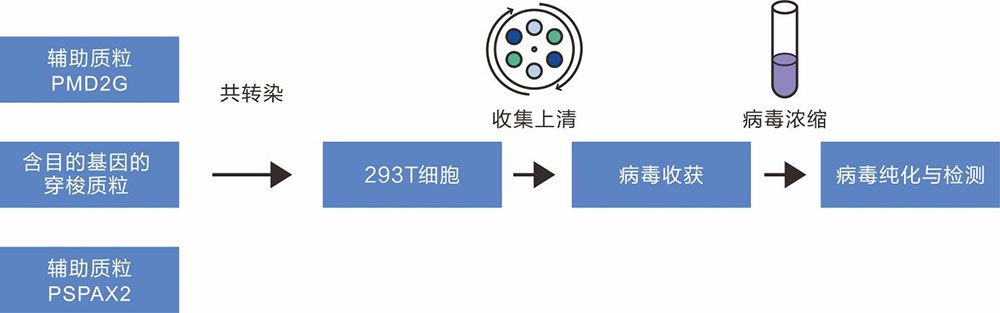
Figure 1 Lentivirus packaging flowchart
1) Select different rLV carriers according to the experimental purpose and the tested cells. The target gene was cloned into LV expression vector and the expression function of the vector was verified by simple enzyme-ligation-transformation-sequencing.
2) In order to obtain plasmid with high quality, high concentration and low endotoxin content, plasmid DNA was purified by column centrifugation and endotoxin detoxification reagents.
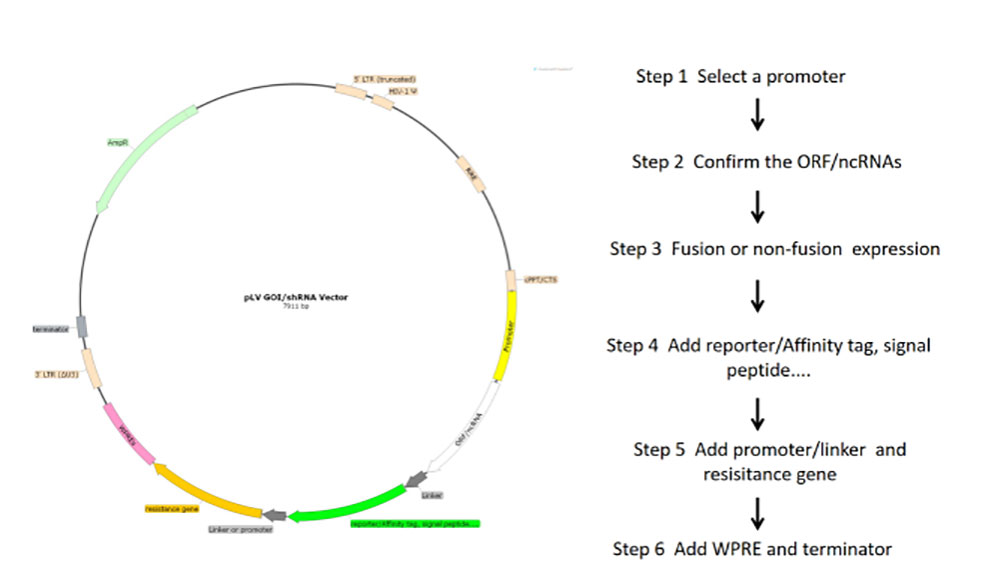
Figure 2 Flowchart of lentivirus core plasmid construction
1 Cell preparation
The frozen cells were removed from liquid nitrogen irrigation and resuscitated in a water bath at 37℃. After centrifugation, fresh medium was added to the cells and cultured at 37℃ and 5%CO2. Cell passage was performed every 2-3 days. After the cells grew normally, they were transferred to a 10cm petri dish for adherent culture.
2 plasmid transfection
Transfection could be performed when the cell density reached a confluence rate of about 80-90%. After the transfection triplasmid system was configured in a certain proportion, the plasmid was transfected into the prepared 293T cells using transfection reagent (PEI max), and transfection and culture were performed according to the system of 1000μL per dish. After transfection, fresh medium was replaced after 6h. (Transfection conditions refer to PEI max transfection reagent use method)
3-cell culture
One day after culture, the cell transfection was observed by microscope.
4 Virus collection and decontamination
After 48 hours of culture, the virus supernatant was collected and continued to be cultured with fresh media. After 72 hours, the virus supernatant was collected and filtered by 0.45μm filter membrane.
5 Virus Purification
The filtered virus supernatant was centrifuged at 25000rpm at 4 ° C for 2.5h. After centrifugation, the supernatant was discarded, the virus precipitation was re-suspended with the virus preservation solution, dissolved at 4 ° C and placed overnight.
6 Virus Collection
Collect and package the venom.
The titer unit of lentivirus is TU/mL, which refers to the number of biologically active virus particles per milliliter, that is, the number of viral genomes that infect and enter the target cell. According to the expression of the target gene after infection, the content of lentivirus in unit volume can be converted, that is, the infection titer of lentivirus.
Detection methods:Virus samples were added to 293T cells in 96-well plates, cultured at 37℃ for 72h, and the growth of bacteria and fungi was detected by microscope.
Test criteria:The medium should be clear and transparent, with no obvious particles in the intercellular space and no bacterial or fungal contamination.
Detection methods:Primers were designed using the conserved region of mycoplasma 16S rRNA gene, and the contamination of mycoplasma in viral samples was quantitatively detected by gel electrophoresis and fluorescence.
Detection criteria:PCR glue map without strip.