AAV ()
Production of recombination adeno-associated virus(rAAV)
The production of recombinant adeno-associated virus was mainly based on the co-transfection of three plasmids (core plasmid pAAV-GOI/shRNA, serotype plasmid pAAV-RC, and helper plasmid pHelper) into HEK293T cells. The packaging cell trans provides the structural protein Cap and the functional protein Rep of rAAV, and with the help of the helper plasmid pHelper, the target DNA fragment with ITR is efficiently packaged into the packaging rAAV virus particles.
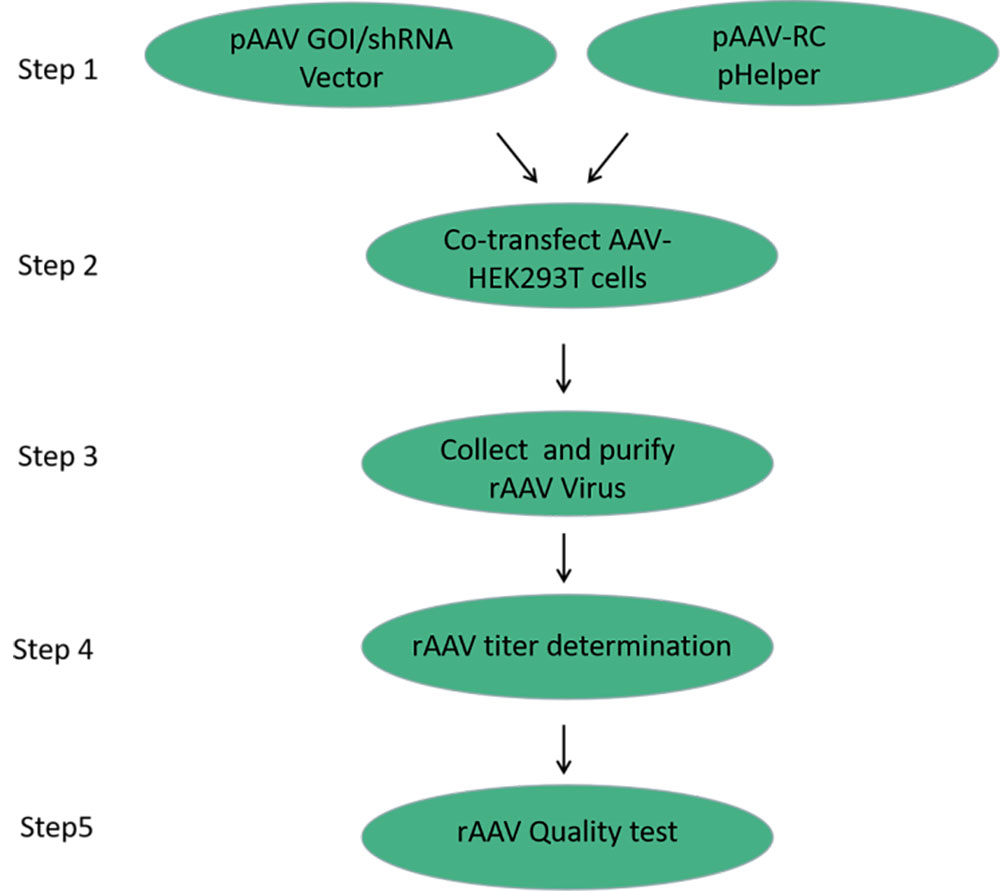
FIG. 1 Production process of rAAV
1.rAAV carrier construction
- 1)Select different rAAV vectors according to the experimental purpose and the tested cells. The target gene was cloned into the rAAV expression vector and the expression function of the vector was verified by a simple enzyme-ligation-transformation-sequencing method.
- 2) In order to obtain plasmid with high quality, high concentration and low endotoxin content, plasmid DNA was purified by column centrifugation and endotoxin detoxification reagents.
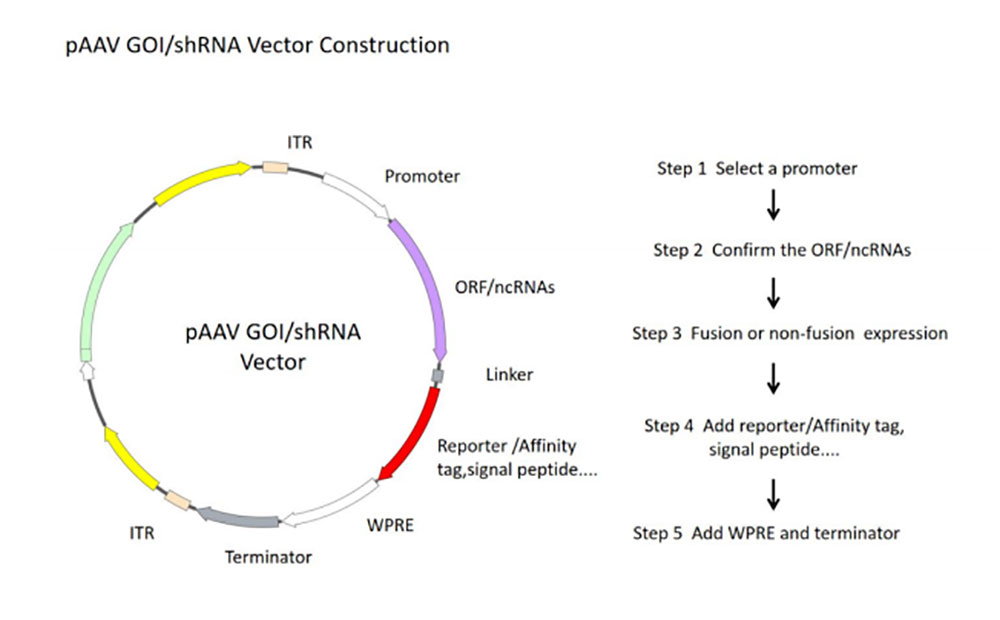
图2 rAAV核心质粒构建流程图
2.rAAV virus packaging
- 1) Cell preparation
The frozen cells were removed from liquid nitrogen irrigation and resuscitated in a water bath at 37℃. After centrifugation, fresh medium was added to the cells and cultured at 37℃ and 5%CO2. Cell passage was performed every 2-3 days. After the cells grew normally, they were transferred to a 10cm petri dish for adherent culture.
- 2) plasmid transfection
When the cell density reached a confluence rate of about 80-90%, transfection could be performed. After the transfection triplasmid system was configured according to a certain proportion, transfection and culture were performed according to the system of 1000μL per dish.
- 3) cell culture
One day after culture, the cell transfection was observed by microscope. In general, the transfection efficiency should be above 80%, and too low transfection rate will lead to decreased virus production.
- 4) Virus collection and decontamination
After 72 hours of culture, the virus supernatant was collected. The virus culture supernatant was centrifuged at low temperature to remove the cell fragments, and an appropriate amount of nuclease was added to remove the free nucleic acid at 37℃, and then the PEG8000/NaCl solution was added to polyprecipitation overnight.
- 5) Virus purification and concentration
The polymerized viruses were re-suspended with PBS and placed in ultra-fast centrifuge for density gradient centrifugation. After centrifugation, the solution of the corresponding layer of the virus was extracted, and the dialysis bag was used to further dialysis at 4℃ overnight. On the second day, the dialysate was filtered by 0.22um, and concentrated and cleaned through the concentration tube to achieve the purpose of impurity removal and concentration.
Quality test of rAAV
The quality of adeno-associated virus mainly includes titer detection, purity detection, sterility detection, mycoplasma detection and endotoxin detection.
- 1 ) Cell preparation
The frozen cells were removed from liquid nitrogen irrigation and resuscitated in a water bath at 37℃. After centrifugation, fresh medium was added to the cells and cultured at 37℃ and 5%CO2. Cell passage was performed every 2-3 days. After the cells grew normally, they were transferred to a 10cm petri dish for adherent culture.
- 2) plasmid transfection
When the cell density reached a confluence rate of about 80-90%, transfection could be performed. After the transfection triplasmid system was configured according to a certain proportion, transfection and culture were performed according to the system of 1000μL per dish.
- 3) cell culture
One day after culture, the cell transfection was observed by microscope. In general, the transfection efficiency should be above 80%, and too low transfection rate will lead to decreased virus production.
- 4) Virus collection and decontamination
After 72 hours of culture, the virus supernatant was collected. The virus culture supernatant was centrifuged at low temperature to remove the cell fragments, and an appropriate amount of nuclease was added to remove the free nucleic acid at 37℃, and then the PEG8000/NaCl solution was added to polyprecipitation overnight.
- 5) Virus purification and concentration
The polymerized viruses were re-suspended with PBS and placed in ultra-fast centrifuge for density gradient centrifugation. After centrifugation, the solution of the corresponding layer of the virus was extracted, and the dialysis bag was used to further dialysis at 4℃ overnight. On the second day, the dialysate was filtered by 0.22um, and concentrated and cleaned through the concentration tube to achieve the purpose of impurity removal and concentration.
Quality test of rAAV
The quality of adeno-associated virus mainly includes titer detection, purity detection, sterility detection, mycoplasma detection and endotoxin detection.
- 1 Titer detection
Detection methods: Complete rAAV virions contain 1 copy of genomic DNA. Virus samples are cleaved acid-base to expose genomic DNA. The DNA copy number of the rAAV virions, i.e., the number of rAAV (Viral genomes), was determined by a fluorescence quantitative PCR experiment using the specific primers, and converted to the rAAV content per unit volume, i.e., the titer of rAAV, i.e., VG/mL (Viral genomes/mL). QC standard: titer ≥1.0E+12 VG/mL
- 2 Purity test
Detection method: The purity of virus samples was determined by SDS-PAGE method. QC standard: In the silver staining results, only VP1, VP2 and VP3 protein stain bands, brightness ratio of about 1:1:10 and no obvious impurity bands.
- 3 Sterility test
Detection methods:The virus samples were inoculated into the medium suitable for the growth of each bacterium and cultured at 37℃ for 7 days to detect the growth of bacteria and fungi.
QC standard:The medium should be clear and transparent, without any bacterial and fungal contamination.
Detection of 4 mycoplasma
Detection methods:Primers were designed using the conserved region of mycoplasma 16S rRNA gene, and the contamination of mycoplasma in viral samples was quantitatively detected by gel electrophoresis and fluorescence.
QC standard:PCR glue map without strip.
- 5 Endotoxin detection
Detection methods:According to the endotoxin detection limulus kit (test tube quantitative chromogenic matrix method) instruction manual.
QC standard: < 10EU/ml

