- E-mail:bd@braincase.cn
- Tel:+86 18971216876
herpes simplex virus (HSV) is one of the most widely used oncolytic viruses (OV). HSV is a double-stranded DNA virus that encodes 84 different peptides with a gene length of 152Kb. HSV genes are divided into three categories according to the timing of their expression: early or α genes, middle or β genes, and late or γ genes. These oncolytic genes encode proteins that promote viral replication and trigger host immune responses [1]. Viral DNA replication and protein synthesis cause cancer cells to lyse, and then daughter viruses are released to infect neighboring cancer cells, expanding their ability to kill cancer. HSV can act locally (peritoneal or pleural perfusion and intratumoral injection) and through systemic pathways (vascular transmission). Intratumoral injection limits its delivery to the tumor site only. Physical barriers such as extracellular matrix and host immune responses to HSV inhibit viral replication.
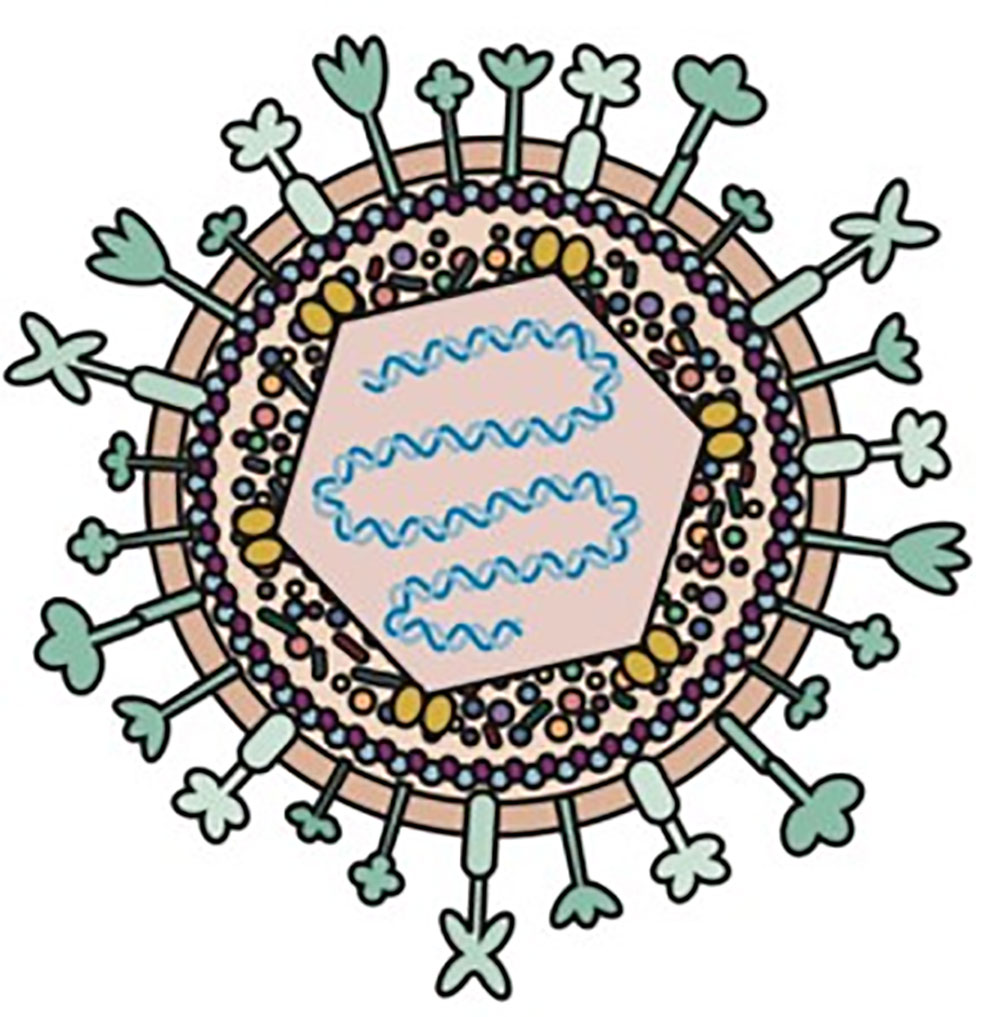
Figure 1. Diagram of Herpes simplex virus type 1 (HSV-1)
oncolytic herpes simplex virus (oHSV) has the following advantages over other oncolytic viruses [2-3] :
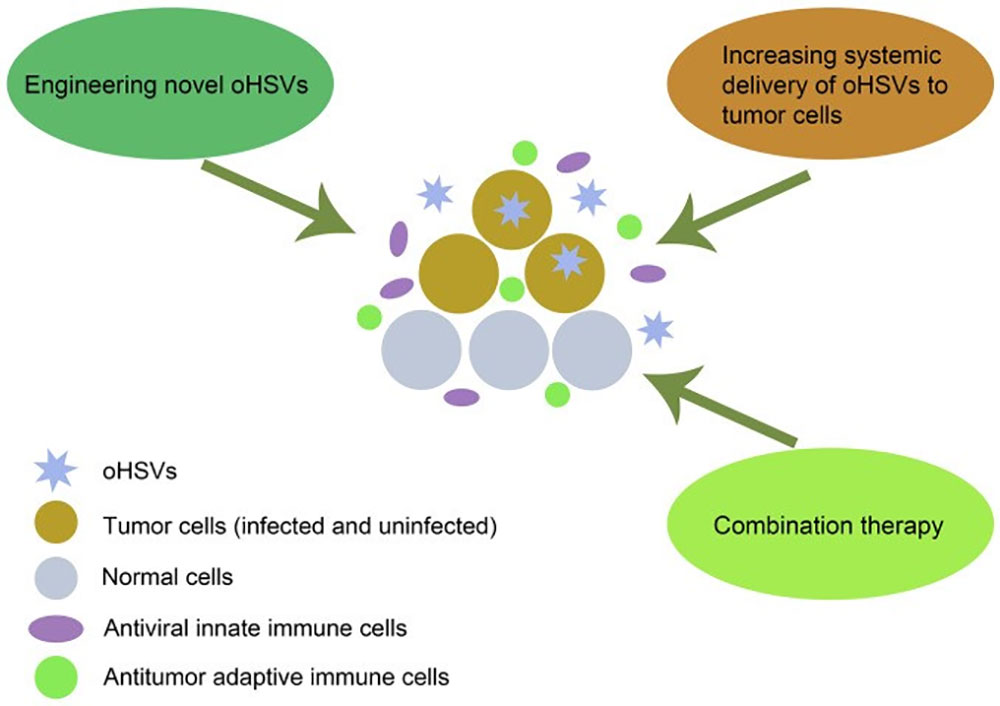
Figure 2: mechanism of oHSVs destroy tumor cells and multiple strategies to improve the curative effect. doi: 10.3892 / ol. 2021.12771
oHSV directly destroys tumor cells by selectively replicating in tumor cells and evading antiviral immunity, and stimulates anti-tumor immune responses, which is the primary mechanism by which oHSV kills cancer cells. Several strategies can improve the efficacy of oHSV in tumor therapy, including designing novel oHSV, increasing the systemic delivery of oHSV to tumor cells, and combination therapy.
In 2015, talimogene laherparepvec(T-VEC), approved by the US Food and Drug Administration (FDA) for the treatment of melanoma, is an effective clinical therapeutic drug formed after the genetic modification of HSV-1, and also a major breakthrough in the clinical application of OV [5-6]. T-VEC, formally known as OncovexGM-CSF, is a genetically engineered strain of HSV-1 that is missing two copies of the ICP34.5 gene and the ICP47 coding gene. The gene encoding human granulocyte-macrophage colonystimulating factor (GM-CSF) was inserted. OncovexGM-CSF performs anti-tumor effects by directly tumor-lysis and inducing tumor-specific secondary immune responses, including enhancing antigen-specific T cell responses and inhibiting levels of CD4+Tregs, CD8+Ts, and bone marrow-derived suppressive immune cells (MDSCs) [7,8]. In 2021, the third-generation oncolytic herpes simplex virus vector G47Δ was approved by the Japanese Ministry of Health, Labour and Welfare (WHLW) for the treatment of gliomas. In order to improve its anti-tumor activity, ICP34.5 gene was knocked out to eliminate the neurotoxicity of the virus, and 312bp α47 gene was knocked out to improve the replication and reproduction of the virus [9-10]. At the same time, the insertion of the ECOL-I LacZ gene into the ICP6 (UL39) region inactivated the nucleotide reductase (RR), allowing the virus to multiply only in tumor cells, thereby improving its oncolytic effect [9]. G47Δ has shown strong replication capacity in a variety of cancers, effectively induces specific anti-tumor immunity, and has shown high safety [10].
At present, HSV gene modification is mostly based on ICP6 gene (UL39) inactivation and ICP34.5 gene deletion [12]. The ICP6 protein is a large subunit of ribonucleotide reductase, which is essential for viral DNA replication. ICP6 mutated HSV can only replicate in rapidly dividing cells such as tumor cells, which can improve its tumor targeting. The viral gene ICP34.5 is the main neurotoxic gene in HSV [11]. As a result of genetic modification, ICP6/ICP34.5 deficient HSV has low pathogenicity to normal tissues and high oncolytic ability to tumor cells [15].
ⅰ. Deletion of genes necessary for replication in non-dividing cells (e.g. UL39, TK, RR), deletion of neurovirulence genes (ICP34.5) and immune escape genes (ICP47);
ⅱ. Immunostimulating factors were inserted to enhance local immunotoxic reactions (such as GM-CSF, PD-1/PD-L1, IL-2, etc.);
ⅲ. Improve the targeting and transcriptional specificity of tumor cells (such as glycoprotein D) [14-15], or by placing foreign genes under the control of tumor-specific promoters (such as IL-13ra2, HER2, EGF-R, etc.) [16-18] to enhance transcriptional targeting and tumor specificity.
At present, the gene design of HSV-1 generally adopts virus strain or plasmid containing virus genome, which is recombined through multiple rounds of homologous recombination technology or bacterial artificial chromosome (BAC) technology to obtain the target virus.
(1)The original virus or virus genome should meet the requirements of clear origin, comprehensive identification (such as morphological examination, genome sequence analysis, etc.), and clear passage history, and it is recommended to compare the genome and amino acid sequence differences with the virus strains collected in the authoritative database.
(2) The purpose and basis of deleting genes or inserting genes should be expounded based on data from literature or validation studies, and studies should be conducted to prove whether the changes in the selective killing of tumor cells, viral infection activity and neurotoxicity reduction of the virus after gene editing meet the theoretical design expectations, and whether additional risks are introduced.
(3) Clearly source of the inserted gene sequence. If a certain gene sequence in a listed product is used, it should be described in detail whether it has undergone gene editing operations and compared with the gene sequence in a listed product. The field of pharmacodynamic evaluation of nervous system diseases can provide you with a one-stop platform from the gene molecular level to the cell tissue level, to the neural circuit, and finally to the animal as a whole behavioral evaluation platform.
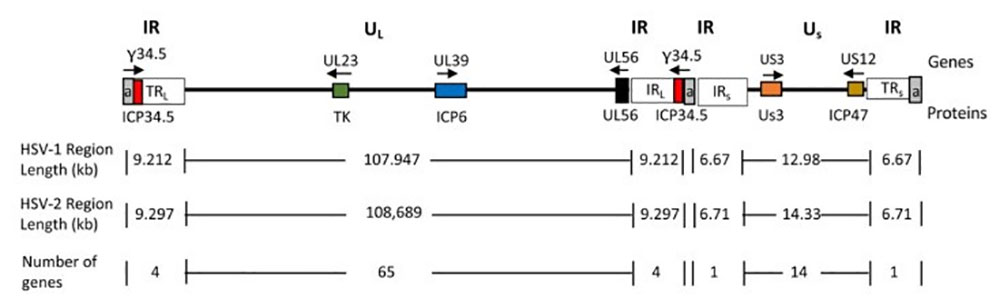
Figure 3. HSV genome diagram. doi:10.3390/v13091740
The times the repeated sequence is (a, gray) is variable. Genes specifically associated with tumors are shown in colored boxes with their names above the genomic lines; Gene product names are shown below the genome line. Arrows indicate the direction of transcription.
BrainCase focuses on viral vector tool research and translational medicine research. Based on years of accumulated experience in gene therapy research and development, we have established a research platform in the field of oncolytic virus vectors, such as plasmid, cell therapy, gene therapy, vector modification, virus preparation, efficacy experiment, etc. At present, BrainCase can supplyconstruction and efficacy services of Adenovirus, Vaccinia Virus, Herpes Simplex Virus, Vesicular Stomatitis virus and other oncolytic virus to customers worldwide.